Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing
- PMID: 16216890
- PMCID: PMC2213212 (VSports注册入口)
- DOI: 10.1084/jem.20051100
VSports最新版本 - Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing
Abstract
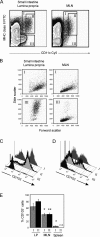
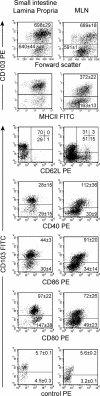
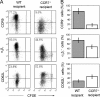
Gut-associated lymphoid tissue (GALT) dendritic cells (DCs) display a unique ability to generate CCR9+alpha4beta7+ gut-tropic CD8+ effector T cells. We demonstrate efficient induction of CCR9 and alpha4beta7 on CD8+ T cells in mesenteric lymph nodes (MLNs) after oral but not intraperitoneal (i VSports手机版. p. ) antigen administration indicating differential targeting of DCs via the oral route. In vitro, lamina propria (LP)-derived DCs were more potent than MLN or Peyer's patch DCs in their ability to generate CCR9+alpha4beta7+ CD8+ T cells. The integrin alpha chain CD103 (alphaE) was expressed on almost all LP DCs, a subset of MLN DCs, but on few splenic DCs. CD103+ MLN DCs were reduced in number in CCR7-/- mice and, although CD8+ T cells proliferated in the MLNs of CCR7-/- mice after i. p. but not oral antigen administration, they failed to express CCR9 and had reduced levels of alpha4beta7. Strikingly, although CD103+ and CD103- MLN DCs were equally potent at inducing CD8+ T cell proliferation and IFN-gamma production, only CD103+ DCs were capable of generating gut-tropic CD8+ effector T cells in vitro. Collectively, these results demonstrate a unique function for LP-derived CD103+ MLN DCs in the generation of gut-tropic effector T cells. .
VSports手机版 - Figures




Comment in
-
VSports注册入口 - Dcs tailor T cells to the tissue.Nat Rev Immunol. 2015 Aug;15(8):469. doi: 10.1038/nri3893. Nat Rev Immunol. 2015. PMID: 26205582 No abstract available.
References
-
- Kunkel, E.J., and E.C. Butcher. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 16:1–4. - PubMed
-
- Svensson, M., J. Marsal, A. Ericsson, L. Carramolino, T. Broden, G. Marquez, and W.W. Agace. 2002. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110:1113–1121. - V体育安卓版 - PMC - PubMed
-
- Calzascia, T., F. Masson, W. Di Berardino-Besson, E. Contassot, R. Wilmotte, M. Aurrand-Lions, C. Ruegg, P.Y. Dietrich, and P.R. Walker. 2005. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 22:175–184. - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- "VSports最新版本" Actions
- V体育官网入口 - Actions
- "V体育平台登录" Actions
- "V体育安卓版" Actions
- "V体育官网入口" Actions
- "V体育ios版" Actions
- "VSports手机版" Actions
- "VSports手机版" Actions
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- "V体育平台登录" Actions
- "V体育官网入口" Actions
Substances (V体育平台登录)
- VSports手机版 - Actions
- V体育官网 - Actions
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources
V体育官网入口 - Full Text Sources
Other Literature Sources
Molecular Biology Databases (VSports手机版)
Research Materials

