Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence
- PMID: 16113291
- PMCID: PMC1231137
- DOI: 10.1128/IAI.73.9.5743-5753.2005 (V体育官网)
Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence
Abstract
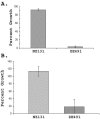
Group A streptococcus (GAS) is a common pathogen of the human skin and mucosal surfaces capable of producing a variety of diseases. In this study, we investigated regulation of iron uptake in GAS and the role of a putative transcriptional regulator named MtsR (for Mts repressor) with homology to the DtxR family of metal-dependent regulatory proteins. An mtsR mutant was constructed in NZ131 (M49 serotype) and analyzed. Western blot and RNA analysis showed that mtsR inactivation results in constitutive transcription of the sia (streptococcal iron acquisition) operon, which was negatively regulated by iron in the parent strain VSports手机版. A recombinant MtsR with C-terminal His(6) tag fusion (rMtsR) was cloned and purified. Electrophoretic mobility gel shift assays demonstrated that rMtsR specifically binds to the sia promoter region in an iron- and manganese-dependent manner. Together, these observations indicate that MtsR directly represses the sia operon during cell growth under conditions of high metal levels. Consistent with deregulation of iron uptake, the mtsR mutant is hypersensitive to streptonigrin and hydrogen peroxide, and (55)Fe uptake assays demonstrate that it accumulates 80% +/- 22. 5% more iron than the wild-type strain during growth in complete medium. Studies with a zebrafish infection model revealed that the mtsR mutant is attenuated for virulence in both the intramuscular and the intraperitoneal routes. In conclusion, MtsR, a new regulatory protein in GAS, controls iron homeostasis and has a role in disease production. .
"VSports手机版" Figures


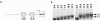


V体育官网入口 - References
-
- Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. - PubMed
-
- Barton, H. A., Z. Johnson, C. D. Cox, A. I. Vasil, and M. L. Vasil. 1996. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol. Microbiol. 21:1001-1017. - PubMed
-
- Bates, C. S., G. E. Montanez, C. R. Woods, R. M. Vincent, and Z. Eichenbaum. 2003. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect. Immun. 71:1042-1055. - VSports最新版本 - PMC - PubMed
-
- Boland, C. A., and W. G. Meijer. 2000. The iron dependent regulatory protein IdeR (DtxR) of Rhodococcus equi. FEMS Microbiol. Lett. 191:1-5. - PubMed
Publication types
- "V体育平台登录" Actions
- Actions (VSports手机版)
MeSH terms (VSports在线直播)
- "V体育安卓版" Actions
- Actions (V体育平台登录)
- "VSports手机版" Actions
- "V体育ios版" Actions
- Actions (VSports注册入口)
- V体育平台登录 - Actions
- VSports最新版本 - Actions
- VSports - Actions
- "VSports注册入口" Actions
- Actions (VSports注册入口)
- VSports - Actions
Substances
- Actions (V体育ios版)
- VSports在线直播 - Actions
"V体育2025版" Grants and funding
LinkOut - more resources
"VSports最新版本" Full Text Sources
Medical

