Detection, epitope-mapping and function of anti-Fas autoantibody in patients with silicosis
- PMID: 16108814
- PMCID: PMC1802403
- DOI: "VSports app下载" 10.1111/j.1365-2567.2005.02192.x
VSports最新版本 - Detection, epitope-mapping and function of anti-Fas autoantibody in patients with silicosis
Abstract
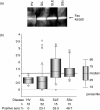
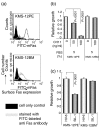
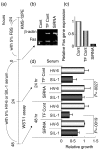
Dysregulation of apoptosis through the Fas-Fas ligand pathway is associated with the onset of autoimmune disease. Since autoantibodies directed against unknown antigens are present in the sera of these patients, sera samples were examined for the presence of autoantibodies directed against the Fas molecule. Using Western blotting and a ProteinChip analysis, autoantibodies against Fas were detected in patients with silicosis, systemic lupus erythematosus (SLE) and systemic sclerosis (SSc), and weakly detected in healthy individuals. Using epitope mapping employing 12-amino-acid polypeptides with the SPOTs system, a minimum of four epitopes and a maximum of 10 epitopes were found. Several amino acid residues involved in binding FasL, such as C66, R87, L90, E93 and H126, were presented within the epitopes. Serum containing a large amount of anti-Fas autoantibody from silicosis patients inhibited the growth of a Fas-expressing human cell line, but did not inhibit the growth of a low Fas-expresser nor a Fas-expresser in which the Fas gene had been silenced by small interference RNA. All epitopes in the intracellular region of Fas were located in the death domain. The possible roles of anti-Fas autoantibody detected in healthy volunteers and patients with silicosis or autoimmune diseases are discussed here. VSports手机版.
Figures



References
-
- Otsuki T, Sakaguchi H, Tomokuni A, et al. Soluble Fas mRNA is dominantly expressed in cases with silicosis. Immunology. 1998;94:258. 10.1046/j.1365-2567.1998.00509.x. - DOI - PMC - PubMed
-
- Tomokuni A, Otsuki T, Sakaguchi H, Isozaki Y, Hyodoh F, Kusaka M, Ueki A. Detection of anti-topoisomerase I autoantibody in patients with silicosis. Environ Health Prev Med. 2002;7:7. V体育2025版 - 10.1265/ehpm.2002.7. - DOI - PMC - PubMed
-
- Ueki H, Kohda M, Nobutoh T, et al. Antidesmoglein autoantibodies in silicosis patients with no bullous diseases. Dermatology. 2001;202:16. 10.1159/000051578. - DOI - PubMed
-
- Ueki A, Isozaki Y, Tomokuni A, et al. Intramolecular epitope spreading among anti-caspase-8 autoantibodies in patients with silicosis, systemic sclerosis and systemic lupus erythematosus, as well as in healthy individuals. Clin Exp Immunol. 2002;129:556. VSports注册入口 - 10.1046/j.1365-2249.2002.01939.x. - DOI - PMC - PubMed
MeSH terms
- "VSports注册入口" Actions
- "VSports" Actions
- Actions (V体育ios版)
- "V体育2025版" Actions
- Actions (VSports在线直播)
- "VSports注册入口" Actions
- Actions (V体育ios版)
- V体育2025版 - Actions
- "V体育安卓版" Actions
- Actions (V体育ios版)
V体育平台登录 - Substances
LinkOut - more resources (V体育安卓版)
Full Text Sources
VSports app下载 - Other Literature Sources
Medical
VSports最新版本 - Molecular Biology Databases
Research Materials
Miscellaneous

