VSports在线直播 - Analysis of the link between enzymatic activity and oligomeric state in AhpC, a bacterial peroxiredoxin
- PMID: 16060667
- PMCID: PMC3832347
- DOI: 10.1021/bi050448i
Analysis of the link between enzymatic activity and oligomeric state in AhpC, a bacterial peroxiredoxin
Abstract
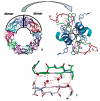
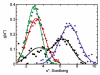
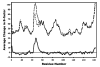
Peroxiredoxins (Prxs) make up a ubiquitous class (proposed EC 1. 11. 1. 15) of cysteine-dependent peroxidases with roles in oxidant protection and signal transduction. An intriguing biophysical property of typical 2-Cys Prxs is the redox-dependent modulation of their oligomeric state between decamers and dimers at physiological concentrations. The functional consequences of this linkage are unknown, but on the basis of structural considerations, we hypothesized that decamer-building (dimer-dimer) interactions serve to stabilize a loop that forms the peroxidatic active site. Here, we address this important issue by studying mutations of Thr77 at the decamer-building interface of AhpC from Salmonella typhimurium. Ultracentrifugation studies revealed that two of the substitutions (T77I and T77D) successfully disrupted the decamer, while the third (T77V) actually enhanced decamer stability. Crystal structures of the decameric forms of all three mutant proteins provide a rationale for their properties. A new assay allowed the first ever measurement of the true k(cat) and K(m) values of wild-type AhpC with H(2)O(2), placing the catalytic efficiency at 4 x 10(7) M(-)(1) s(-)(1). T77V had slightly higher activity than wild-type enzyme, and both T77I and T77D exhibited ca. 100-fold lower catalytic efficiency, indicating that the decameric structure is quite important for, but not essential to, activity. The interplay between decamer formation and active site loop dynamics is emphasized by a decreased susceptibility of T77I and T77D to peroxide-mediated inactivation, and by an increase in the crystallographic B-factors in the active site loop, rather than at the site of the mutation, in the T77D variant VSports手机版. .
Figures


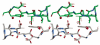
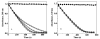

References
-
- Poole LB, Godzik A, Nayeem A, Schmitt JD. AhpF can be dissected into two functional units: Tandem repeats of two thioredoxin-like folds in the N-terminus mediate electron transfer from the thioredoxin reductase-like C-terminus to AhpC. Biochemistry. 2000;39:6602–6615. - VSports在线直播 - PubMed
-
- Hofmann B, Hecht H-J, Flohé L. Peroxiredoxins. Biol. Chem. 2002;383:347–364. - PubMed
-
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. - VSports - PubMed
-
- Wood ZA, Schroder E, Harris JR, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. - VSports - PubMed
-
- Poole LB, Reynolds CM, Wood ZA, Karplus PA, Ellis HR, Li Calzi M. AhpF and other NADH: peroxiredoxin oxidoreductases, homologues of low Mr thioredoxin reductase. Eur. J. Biochem. 2000;267:6126–6133. - VSports最新版本 - PubMed
Publication types
- "VSports手机版" Actions
- "V体育官网入口" Actions
MeSH terms
- VSports手机版 - Actions
- V体育ios版 - Actions
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- "V体育2025版" Actions
- V体育官网 - Actions
- "VSports在线直播" Actions
- VSports手机版 - Actions
- "VSports在线直播" Actions
- VSports - Actions
- "V体育官网入口" Actions
- VSports - Actions
- "VSports" Actions
Substances
- Actions (VSports注册入口)
- V体育安卓版 - Actions
- "VSports手机版" Actions
Associated data
- Actions
- "V体育安卓版" Actions
- "V体育安卓版" Actions
Grants and funding (V体育平台登录)
LinkOut - more resources
Full Text Sources (VSports最新版本)
Other Literature Sources
Research Materials
Miscellaneous

