Tumour necrosis factor alpha induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor kappaB and activator protein-1
- PMID: 16011481
- PMCID: PMC1276939
- DOI: 10.1042/BJ20050795
Tumour necrosis factor alpha induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor kappaB and activator protein-1
Abstract
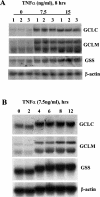
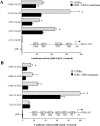
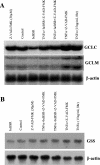
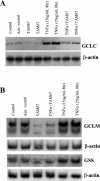
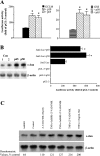
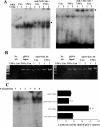
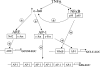
GSH synthesis occurs via two enzymatic steps catalysed by GCL [glutamate-cysteine ligase, made up of GCLC (GCL catalytic subunit), and GCLM (GCL modifier subunit)] and GSS (GSH synthetase). Co-ordinated up-regulation of GCL and GSS further enhances GSH synthetic capacity. The present study examined whether TNFalpha (tumour necrosis factor alpha) influences the expression of rat GSH synthetic enzymes. To facilitate transcriptional studies of the rat GCLM, we cloned its 1 VSports手机版. 8 kb 5'-flanking region. TNFalpha induces the expression and recombinant promoter activities of GCLC, GCLM and GSS in H4IIE cells. TNFalpha induces NF-kappaB (nuclear factor kappaB) and AP-1 (activator protein 1) nuclear-binding activities. Blocking AP-1 with dominant negative c-Jun or NF-kappaB with IkappaBSR (IkappaB super-repressor, where IkappaB stands for inhibitory kappaB) lowered basal expression and inhibited the TNFalpha-mediated increase in mRNA levels of all three genes. While all three genes have multiple AP-1-binding sites, only GCLC has a NF-kappaB-binding site. Overexpression with p50 or p65 increased c-Jun mRNA levels, c-Jun-dependent promoter activity and the promoter activity of GCLM and GSS. Blocking NF-kappaB also lowered basal c-Jun expression and blunted the TNFalpha-mediated increase in c-Jun mRNA levels. TNFalpha treatment resulted in increased c-Jun and Nrf2 (nuclear factor erythroid 2-related factor 2) nuclear binding to the antioxidant response element of the rat GCLM and if this was prevented, TNFalpha no longer induced the GCLM promoter activity. In conclusion, both c-Jun and NF-kappaB are required for basal and TNFalpha-mediated induction of GSH synthetic enzymes in H4IIE cells. While NF-kappaB may exert a direct effect on the GCLC promoter, it induces the GCLM and GSS promoters indirectly via c-Jun. .
Figures (VSports手机版)




References
-
- Lu S. C. Regulation of hepatic glutathione synthesis: current concept and controversies. FASEB J. 1999;13:1169–1183. - PubMed (VSports最新版本)
-
- Poot M., Teubert H., Rabinovitch P. S., Kavanagh T. J. De novo synthesis of glutathione is required for both entry into and progression through the cell cycle. J. Cell. Physiol. 1995;163:555–560. - PubMed
-
- Richman P. G., Meister A. Regulation of γ-glutamylcysteine synthetase by nonallosteric feedback inhibition by glutathione. J. Biol. Chem. 1975;250:1422–1426. - PubMed
-
- Yan N., Meister A. Amino acid sequence of rat kidney γ-glutamylcysteine synthetase. J. Biol. Chem. 1990;265:1588–1593. - V体育ios版 - PubMed
V体育平台登录 - Publication types
- Actions (VSports注册入口)
MeSH terms
- Actions (V体育安卓版)
- "VSports最新版本" Actions
- "V体育官网入口" Actions
- VSports手机版 - Actions
- Actions (V体育官网入口)
- Actions (V体育平台登录)
- V体育安卓版 - Actions
- V体育官网 - Actions
- Actions (VSports注册入口)
Substances
- V体育平台登录 - Actions
- VSports - Actions
VSports - Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
VSports app下载 - Research Materials
Miscellaneous

