Defective thrombus formation in mice lacking coagulation factor XII
- PMID: 16009717
- PMCID: PMC2213000
- DOI: 10.1084/jem.20050664
Defective thrombus formation in mice lacking coagulation factor XII
Abstract
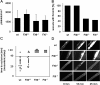
Blood coagulation is thought to be initiated by plasma protease factor VIIa in complex with the membrane protein tissue factor. In contrast, coagulation factor XII (FXII)-mediated fibrin formation is not believed to play an important role for coagulation in vivo. We used FXII-deficient mice to study the contributions of FXII to thrombus formation in vivo. Intravital fluorescence microscopy and blood flow measurements in three distinct arterial beds revealed a severe defect in the formation and stabilization of platelet-rich occlusive thrombi. Although FXII-deficient mice do not experience spontaneous or excessive injury-related bleeding, they are protected against collagen- and epinephrine-induced thromboembolism VSports手机版. Infusion of human FXII into FXII-null mice restored injury-induced thrombus formation. These unexpected findings change the long-standing concept that the FXII-induced intrinsic coagulation pathway is not important for clotting in vivo. The results establish FXII as essential for thrombus formation, and identify FXII as a novel target for antithrombotic therapy. .
Figures (V体育官网)
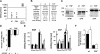
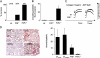
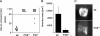
Comment in
-
Are hemostasis and thrombosis two sides of the same coin?J Exp Med. 2006 Mar 20;203(3):493-5. doi: 10.1084/jem.20060217. Epub 2006 Mar 13. J Exp Med. 2006. PMID: 16533890 Free PMC article.
V体育官网入口 - References
-
- Macfarlane, R.G. 1964. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 202:498–499. - PubMed
-
- Davie, E.W., and O.D. Ratnoff. 1964. Waterfall sequence for intrinsic blood clotting. Science. 145:1310–1312. - PubMed (V体育平台登录)
-
- Ruggeri, Z.M. 2002. Platelets in atherothrombosis. Nat. Med. 8:1227–1234. - PubMed
-
- Mackman, N. 2004. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler. Thromb. Vasc. Biol. 24:1015–1022. - V体育2025版 - PubMed
-
- Cochrane, C.G., and J.H. Griffin. 1982. The biochemistry and pathophysiology of the contact system of plasma. Adv. Immunol. 33:241–306. - PubMed
Publication types
MeSH terms
- V体育2025版 - Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- Actions (VSports app下载)
- Actions (V体育2025版)
- "V体育官网入口" Actions
- "V体育官网" Actions
- "V体育2025版" Actions
- Actions (VSports app下载)
- V体育官网 - Actions
- Actions (VSports在线直播)
Substances
- VSports在线直播 - Actions
- Actions (V体育安卓版)
"V体育平台登录" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

