Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells
- PMID: 15931392
- PMCID: PMC1137001
- DOI: 10.1172/JCI24480
"VSports注册入口" Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells
Abstract
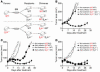
T cell differentiation is a progressive process characterized by phenotypic and functional changes. By transferring tumor-specific CD8+ T cells into tumor-bearing mice at various stages of differentiation, we evaluated their efficacy for adoptive immunotherapy VSports手机版. We found that administration of naive and early effector T cells, in combination with active immunization and IL-2, resulted in the eradication of large, established tumors. Despite enhanced in vitro antitumor properties, more-differentiated effector T cells were less effective for in vivo tumor treatment. Several events may underlie this paradoxical phenomenon: (a) downregulation of lymphoid-homing and costimulatory molecules; (b) inability to produce IL-2 and access homeostatic cytokines; and (c) entry into a proapoptotic and replicative senescent state. While the progressive acquisition of terminal effector properties is characterized by pronounced in vitro tumor killing, in vivo T cell activation, proliferation, and survival are progressively impaired. These findings suggest that the current methodology for selecting T cells for transfer is inadequate and provide new criteria for the generation and the screening of optimal lymphocyte populations for adoptive immunotherapy. .
VSports app下载 - Figures




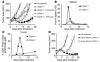


Comment in
-
Toward improved immunocompetence of adoptively transferred CD8+ T cells.J Clin Invest. 2005 Jun;115(6):1467-9. doi: 10.1172/JCI25427. J Clin Invest. 2005. PMID: 15931384 Free PMC article.
References
-
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. - VSports注册入口 - PMC - PubMed
-
- Childs RW, Barrett J. Nonmyeloablative allogeneic immunotherapy for solid tumors. Annu. Rev. Med. 2004;55:459–475. - PubMed
-
- Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. - PMC (VSports) - PubMed
MeSH terms
- "V体育安卓版" Actions
- V体育2025版 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- Actions (V体育官网)
Substances
- Actions (VSports最新版本)
Grants and funding
LinkOut - more resources
V体育ios版 - Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育官网)
Research Materials

