Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients
- PMID: 15867097
- PMCID: PMC1389847 (V体育ios版)
- DOI: "V体育ios版" 10.1084/jem.20042592
Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients
Erratum in
- J Exp Med. 2007 Oct;204(10):2487
Abstract
Natural killer T (NKT) cells are distinct glycolipid reactive innate lymphocytes that are implicated in the resistance to pathogens and tumors. Earlier attempts to mobilize NKT cells, specifically, in vivo in humans met with limited success. Here, we evaluated intravenous injection of monocyte-derived mature DCs that were loaded with a synthetic NKT cell ligand, alpha-galactosyl-ceramide (alpha-GalCer; KRN-7000) in five patients who had advanced cancer. Injection of alpha-GalCer-pulsed, but not unpulsed, dendritic cells (DCs) led to >100-fold expansion of several subsets of NKT cells in all patients; these could be detected for up to 6 mo after vaccination. NKT activation was associated with an increase in serum levels of interleukin-12 p40 and IFN-gamma inducible protein-10 VSports手机版. In addition, there was an increase in memory CD8+ T cells specific for cytomegalovirus in vivo in response to alpha-GalCer-loaded DCs, but not unpulsed DCs. These data demonstrate the feasibility of sustained expansion of NKT cells in vivo in humans, including patients who have advanced cancer, and suggest that NKT activation might help to boost adaptive T cell immunity in vivo. .
Figures
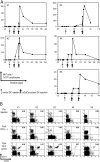






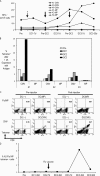

"VSports最新版本" Comment in
-
Research on human subjects in the JEM.J Exp Med. 2005 May 2;201(9):1349-50. doi: 10.1084/jem.20050723. J Exp Med. 2005. PMID: 15867088 Free PMC article. No abstract available.
References
-
- Godfrey, D.I., and M. Kronenberg. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114:1379–1388. - "V体育ios版" PMC - PubMed
-
- Brigl, M., and M.B. Brenner. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817–890. - PubMed
-
- Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 278:1626–1629. - PubMed
-
- Stetson, D.B., M. Mohrs, R.L. Reinhardt, J.L. Baron, Z.E. Wang, L. Gapin, M. Kronenberg, and R.M. Locksley. 2003. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198:1069–1076. - V体育安卓版 - PMC - PubMed
-
- Eberl, G., and H.R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985–992. - PubMed
Publication types
- Actions (V体育官网)
MeSH terms
- V体育官网 - Actions
- "VSports手机版" Actions
- V体育安卓版 - Actions
- "V体育2025版" Actions
- Actions (VSports)
- VSports最新版本 - Actions
- V体育安卓版 - Actions
Substances
- "VSports app下载" Actions
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- "VSports注册入口" Actions
- Actions (VSports app下载)
Grants and funding
LinkOut - more resources
"V体育2025版" Full Text Sources
Other Literature Sources
Medical
Research Materials
"V体育2025版" Miscellaneous

