Molecular and ionic mimicry and the transport of toxic metals
- PMID: 15845419
- PMCID: PMC2409291
- DOI: VSports - 10.1016/j.taap.2004.09.007
Molecular and ionic mimicry and the transport of toxic metals (V体育平台登录)
Abstract
Despite many scientific advances, human exposure to, and intoxication by, toxic metal species continues to occur. Surprisingly, little is understood about the mechanisms by which certain metals and metal-containing species gain entry into target cells. Since there do not appear to be transporters designed specifically for the entry of most toxic metal species into mammalian cells, it has been postulated that some of these metals gain entry into target cells, through the mechanisms of ionic and/or molecular mimicry, at the site of transporters of essential elements and/or molecules. The primary purpose of this review is to discuss the transport of selective toxic metals in target organs and provide evidence supporting a role of ionic and/or molecular mimicry. In the context of this review, molecular mimicry refers to the ability of a metal ion to bond to an endogenous organic molecule to form an organic metal species that acts as a functional or structural mimic of essential molecules at the sites of transporters of those molecules. Ionic mimicry refers to the ability of a cationic form of a toxic metal to mimic an essential element or cationic species of an element at the site of a transporter of that element. Molecular and ionic mimics can also be sub-classified as structural or functional mimics. This review will present the established and putative roles of molecular and ionic mimicry in the transport of mercury, cadmium, lead, arsenic, selenium, and selected oxyanions in target organs and tissues VSports手机版. .
VSports app下载 - Figures

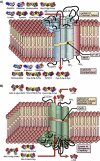
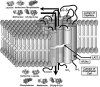
References
-
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000;275:19906–19912. - VSports app下载 - PubMed
-
- Afonso J, deAlvarez R. Effects of mercury on human gestation. Am. J. Obstet. Gynecol. 1960;80:145–154. - "V体育平台登录" PubMed
-
- Agency for Toxic Substance and Disease Registry . Toxicological Profile for Mercury. U.S. Department of Health and Humans Services, Public Health Service, Centers for Disease Control; Atlanta, GA: 2003a.
-
- Agency for Toxic Substance and Disease Registry . Toxicological Profile for Cadmium. U.S. Department of Health and Humans Services, Public Health Service, Centers for Disease Control; Atlanta, GA: 2003b.
-
- Agency for Toxic Substance and Disease Registry . Toxicological Profile for Lead. U.S. Department of Health and Humans Services, Public Health Service, Centers for Disease Control; Atlanta, GA: 2003c.
Publication types
- "V体育官网入口" Actions
VSports最新版本 - MeSH terms
- "V体育ios版" Actions
Substances
- "V体育2025版" Actions
- "VSports注册入口" Actions
- Actions (V体育安卓版)
- VSports注册入口 - Actions

