Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities (VSports)
- PMID: 15809227
- PMCID: VSports手机版 - PMC1074398
- DOI: 10.1093/nar/gki343
Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities
Abstract
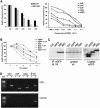
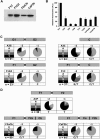
Human APOBEC3F and APOBEC3G are double-domained deaminases that can catalyze dC-->dU deamination in HIV-1 and MLV retroviral DNA replication intermediates, targeting T-C or C-C dinucleotides, respectively. HIV-1 antagonizes their action through its vif gene product, which has been shown (at least in the case of APOBEC3G) to interact with the N-terminal domain of the deaminase, triggering its degradation. Here, we compare APOBEC3F and APOBEC3G to APOBEC3C, a single-domained deaminase that can also act on both HIV-1 and MLV. We find that whereas APOBEC3C contains all the information necessary for both Vif-binding and cytidine deaminase activity in a single domain, it is the C-terminal domain of APOBEC3F and APOBEC3G that confer their target site specificity for cytidine deamination VSports手机版. We have exploited the fact that APOBEC3C, whilst highly homologous to the C-terminal domain of APOBEC3F, exhibits a distinct target site specificity (preferring Y-C dinucleotides) in order to identify residues in APOBEC3F that might affect its target site specificity. We find that this specificity can be altered by single amino acid substitutions at several distinct positions, suggesting that the strong dependence of APOBEC3-mediated deoxycytidine deamination on the 5'-flanking nucleotide is sensitive to relatively subtle changes in the APOBEC3 structure. The approach has allowed the isolation of APOBEC3 DNA mutators that exhibit novel target site preferences. .
Figures


V体育官网 - References
-
- Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. - PubMed
-
- Marin M., Rose K.M., Kozak S.L., Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nature Med. 2003;9:1398–1403. - PubMed
-
- Stopak K., de Noronha C., Yonemoto W., Greene W.C. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell. 2003;12:591–601. - PubMed
-
- Sheehy A.M., Gaddis N.C., Malim M.H. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nature Med. 2003;9:1404–1407. - PubMed
-
- Yu X., Yu Y., Liu B., Luo K., Kong W., Mao P., Yu X.F. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. - PubMed (VSports)
Publication types
V体育ios版 - MeSH terms
- Actions (VSports手机版)
- Actions (V体育官网入口)
- "VSports" Actions
- V体育安卓版 - Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
- "V体育官网" Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
Substances (VSports最新版本)
- Actions (V体育平台登录)
- "V体育官网" Actions
- VSports最新版本 - Actions
- Actions (V体育平台登录)
LinkOut - more resources
Full Text Sources
Other Literature Sources

