Critical role of host gammadelta T cells in experimental acute graft-versus-host disease
- PMID: 15797996
- PMCID: PMC1895173
- DOI: 10.1182/blood-2004-10-4087
Critical role of host gammadelta T cells in experimental acute graft-versus-host disease
Abstract (V体育官网入口)
Gammadelta T cells localize to target tissues of graft-versus-host disease (GVHD) and therefore we investigated the role of host gammadelta T cells in the pathogenesis of acute GVHD in several well-characterized allogeneic bone marrow transplantation (BMT) models. Depletion of host gammadelta T cells in wild-type (wt) B6 recipients by administration of anti-T-cell receptor (TCR) gammadelta monoclonal antibody reduced GVHD, and gammadelta T-cell-deficient (gammadelta-/-) BM transplant recipients experienced markedly improved survival compared with normal controls (63% vs 10%, P < . 001). gammadelta T cells were responsible for this difference because reconstitution of gammadelta-/- recipients with gammadelta T cells restored GVHD mortality. gammadelta-/- recipients showed decreased serum levels of tumor necrosis factor alpha (TNF-alpha), less GVHD histopathologic damage, and reduced donor T-cell expansion. Mechanistic analysis of this phenomenon demonstrated that dendritic cells (DCs) from gammadelta-/- recipients exhibited less allostimulatory capacity compared to wt DCs after irradiation. Normal DCs derived from BM caused greater allogeneic T-cell proliferation when cocultured with gammadelta T cells than DCs cocultured with medium alone. This enhancement did not depend on interferon gamma (IFN-gamma), TNF-alpha, or CD40 ligand but did depend on cell-to-cell contact. These data demonstrated that the host gammadelta T cells exacerbate GVHD by enhancing the allostimulatory capacity of host antigen-presenting cells. VSports手机版.
Figures

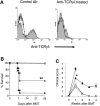

 , n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1 and the gastrointestinal tract was analyzed on day 7 after BMT as described in “Materials and methods.” Damage to syn small bowel was minimal (A), whereas wt exhibited severe villous blunting, crypt destruction changes and atrophy, and increased lymphocytic infiltrates (B). γδ-/- small bowel showed significantly less damage (C). Original magnification, ×100. Images were visualized using an Olympus Bx 40 microscope (Olympus, Melville, NY) equipped with an 10 ×/0.65 aperture objective lens. Image acquisition was performed with a JVC digital camera GC-Qx 5HDU (JVC, Wayne, NJ). Coded slides were scored for pathologic damage (D) as described in “Materials and methods.” Serum was obtained on day 7 after BMT and analyzed for LPS (E) and TNF-α (F). γδ-/- vs wt, *P < .05 (D-F). Error bars represent standard error.
, n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1 and the gastrointestinal tract was analyzed on day 7 after BMT as described in “Materials and methods.” Damage to syn small bowel was minimal (A), whereas wt exhibited severe villous blunting, crypt destruction changes and atrophy, and increased lymphocytic infiltrates (B). γδ-/- small bowel showed significantly less damage (C). Original magnification, ×100. Images were visualized using an Olympus Bx 40 microscope (Olympus, Melville, NY) equipped with an 10 ×/0.65 aperture objective lens. Image acquisition was performed with a JVC digital camera GC-Qx 5HDU (JVC, Wayne, NJ). Coded slides were scored for pathologic damage (D) as described in “Materials and methods.” Serum was obtained on day 7 after BMT and analyzed for LPS (E) and TNF-α (F). γδ-/- vs wt, *P < .05 (D-F). Error bars represent standard error.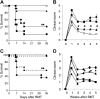
 , n = 12), αβ+ splenocytes (
, n = 12), αβ+ splenocytes ( , n = 8) or media (▴ and solid line, n = 8) were injected into γδ-/- (60 × 106 cells/mouse). Wt B6 mice (• and solid line, n = 12) were injected with media alone. Twenty-four hours later, all mice received transplants from allogeneic BALB/c donors as in Figure 1 and were evaluated for survival (C) and clinical GVHD score (D). Data from 2 similar experiments are combined.
, n = 8) or media (▴ and solid line, n = 8) were injected into γδ-/- (60 × 106 cells/mouse). Wt B6 mice (• and solid line, n = 12) were injected with media alone. Twenty-four hours later, all mice received transplants from allogeneic BALB/c donors as in Figure 1 and were evaluated for survival (C) and clinical GVHD score (D). Data from 2 similar experiments are combined.  vs
vs  , **P < .01 by Wilcoxon rank test.
, **P < .01 by Wilcoxon rank test.  vs
vs  , weeks 3 to 5, *P < .001 by Mann-Whitney U test. Error bars represent standard error.
, weeks 3 to 5, *P < .001 by Mann-Whitney U test. Error bars represent standard error.
 , n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1. Splenocytes were harvested on day 7 after BMT, and donor (H2Dd+) CD4+ T-cell (A) and CD8+ T-cell (B) phenotype were determined by FACS analysis as described in “Materials and methods.” Data represent the mean ± SE. wt vs γδ-/-, *P < .05. (C-E) Splenic DCs were isolated from γδ-/- (▴) and wt (•) mice 6 hours after 1100 cGy TBI and were cultured for 3 to 4 days with allogeneic responder BALB/c T cells. During the final 12 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation (C). Supernatants were collected at 48 hours and 72 hours and assayed by ELISA for IL-2 (D) and IFN-γ (E), respectively, as described in “Materials and methods.” Each graph represents 1 of 4 similar experiments. wt vs γδ-/-, *P < .05. Error bars represent standard error.
, n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1. Splenocytes were harvested on day 7 after BMT, and donor (H2Dd+) CD4+ T-cell (A) and CD8+ T-cell (B) phenotype were determined by FACS analysis as described in “Materials and methods.” Data represent the mean ± SE. wt vs γδ-/-, *P < .05. (C-E) Splenic DCs were isolated from γδ-/- (▴) and wt (•) mice 6 hours after 1100 cGy TBI and were cultured for 3 to 4 days with allogeneic responder BALB/c T cells. During the final 12 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation (C). Supernatants were collected at 48 hours and 72 hours and assayed by ELISA for IL-2 (D) and IFN-γ (E), respectively, as described in “Materials and methods.” Each graph represents 1 of 4 similar experiments. wt vs γδ-/-, *P < .05. Error bars represent standard error.

 vs ▪, *P < .05. (B) TNF-α. □ vs ▪, *P < .05. ND indicates not detected.
vs ▪, *P < .05. (B) TNF-α. □ vs ▪, *P < .05. ND indicates not detected.Comment in
-
Recipient gammadelta T cells in graft-versus-host disease.Blood. 2006 May 1;107(9):3808-9; author reply 3809. doi: 10.1182/blood-2005-11-4586. Blood. 2006. PMID: 16627764 No abstract available.
References
-
- Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285: 412-415. - PubMed
-
- Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172: 7393-7398. - PubMed
-
- Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156: 145-166. - PubMed
Publication types
- "V体育官网入口" Actions
"VSports最新版本" MeSH terms
- "V体育平台登录" Actions
- V体育ios版 - Actions
- V体育官网 - Actions
- Actions (V体育官网)
- Actions (V体育官网)
- "VSports注册入口" Actions
- Actions (VSports在线直播)
- "V体育安卓版" Actions
- "V体育官网" Actions
- V体育官网入口 - Actions
- VSports注册入口 - Actions
- VSports app下载 - Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- V体育官网 - Actions
Substances (V体育2025版)
- V体育安卓版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育官网" Other Literature Sources
V体育ios版 - Molecular Biology Databases
Research Materials (VSports app下载)

