Lack of TCF2/vHNF1 in mice leads to pancreas agenesis
- PMID: 15668393
- PMCID: PMC547822 (VSports)
- DOI: 10.1073/pnas.0405776102 (V体育2025版)
Lack of TCF2/vHNF1 in mice leads to pancreas agenesis
"V体育官网入口" Abstract
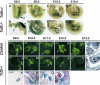
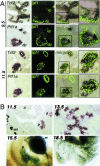
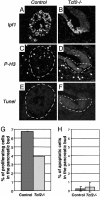
Heterozygous mutations in the human POU-homeobox TCF2 (vHNF1, HNF1beta) gene are associated with maturity-onset diabetes of the young, type 5, and abnormal urogenital tract development VSports手机版. Recently, pancreas atrophies have been reported in several maturity-onset diabetes of the young type 5 patients, suggesting that TCF2 is required not only for adult pancreas function but also for its normal development. Tcf2-deficient mice die before gastrulation because of defective visceral endoderm formation. To investigate the role of this factor in pancreas development, we rescued this early lethality by tetraploid aggregation. We show that TCF2 has an essential function in the first steps of pancreas development, correlated with its expression domain that demarcates the entire pancreatic buds from the earliest stages. Lack of TCF2 results in pancreas agenesis by embryonic day 13. 5. At earlier stages, only a dorsal bud rudiment forms transiently and expresses the transcription factors Ipf1 and Hlxb9 but lacks the key transcription factor involved in the acquisition of a pancreatic fate, Ptf1a, as well as all endocrine precursor cells. Regional specification of the gut also is perturbed in Tcf2-/- embryos as manifested by ectopic expression of Shh and lack of Ihh and Ipf1 in the posterior stomach and duodenum. Our results highlight the requirement of Tcf2 for ensuring both accurate expression of key regulator molecules in the stomach-duodenal epithelium and proper acquisition of the pancreatic fate. This study provides further insights into early molecular events controlling pancreas development and may contribute to the development of cell-replacement strategies for diabetes. .
Figures

References
-
- Apelqvist, A., Ahlgren, U. & Edlund, H. (1997) Curr. Biol. 7, 801-804. - PubMed (VSports注册入口)
-
- Kim, S. K., Hebrok, M. & Melton, D. A. (1997) Development (Cambridge, U.K.) 124, 4243-4252. - "VSports在线直播" PubMed
-
- Gu, G., Dubauskaite, J. & Melton, D. A. (2002) Development (Cambridge, U.K.) 129, 2447-2457. - PubMed
Publication types
VSports app下载 - MeSH terms
- Actions (V体育2025版)
- "V体育官网" Actions
- V体育平台登录 - Actions
- Actions (VSports在线直播)
- VSports app下载 - Actions
- V体育官网 - Actions
- VSports在线直播 - Actions
- VSports app下载 - Actions
- Actions (VSports在线直播)
- Actions (VSports在线直播)
- "V体育官网" Actions
Substances (VSports在线直播)
- Actions (V体育安卓版)
- V体育ios版 - Actions
- "V体育官网入口" Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

