Type V protein secretion pathway: the autotransporter story
- PMID: 15590781
- PMCID: PMC539010
- DOI: "V体育官网" 10.1128/MMBR.68.4.692-744.2004
Type V protein secretion pathway: the autotransporter story (VSports手机版)
Abstract
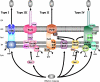
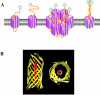
Gram-negative bacteria possess an outer membrane layer which constrains uptake and secretion of solutes and polypeptides. To overcome this barrier, bacteria have developed several systems for protein secretion. The type V secretion pathway encompasses the autotransporter proteins, the two-partner secretion system, and the recently described type Vc or AT-2 family of proteins. Since its discovery in the late 1980s, this family of secreted proteins has expanded continuously, due largely to the advent of the genomic age, to become the largest group of secreted proteins in gram-negative bacteria. Several of these proteins play essential roles in the pathogenesis of bacterial infections and have been characterized in detail, demonstrating a diverse array of function including the ability to condense host cell actin and to modulate apoptosis. However, most of the autotransporter proteins remain to be characterized VSports手机版. In light of new discoveries and controversies in this research field, this review considers the autotransporter secretion process in the context of the more general field of bacterial protein translocation and exoprotein function. .
Figures







References
-
- Ait-Tahar, K., K. G. Wooldridge, D. P. Turner, M. Atta, I. Todd, and D. A. Ala'Aldeen. 2000. Auto-transporter A protein of Neisseria meningitidis: a potent CD4+ T-cell and B-cell stimulating antigen detected by expression cloning. Mol. Microbiol. 37:1094-1105. - PubMed
-
- Aldridge, P., and K. T. Hughes. 2001. How and when are substrates selected for type III secretion? Trends Microbiol. 9:209-214. - VSports最新版本 - PubMed
-
- Al-Hasani, K., I. R. Henderson, H. Sakellaris, K. Rajakumar, T. Grant, J. P. Nataro, R. Robins-Browne, and B. Adler. 2000. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68:2457-2463. - PMC - PubMed
Publication types
MeSH terms
- "VSports" Actions
- Actions (V体育ios版)
Substances
LinkOut - more resources
V体育平台登录 - Full Text Sources
Other Literature Sources
Molecular Biology Databases

