Human cytomegalovirus-specific CD4+-T-cell cytokine response induces fractalkine in endothelial cells
- PMID: 15542669
- PMCID: PMC525022
- DOI: 10.1128/JVI.78.23.13173-13181.2004
Human cytomegalovirus-specific CD4+-T-cell cytokine response induces fractalkine in endothelial cells (VSports注册入口)
Abstract
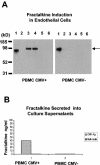
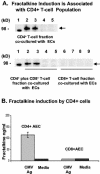
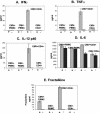
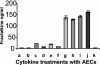
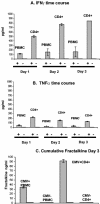
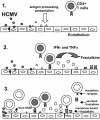
Cytomegalovirus (CMV) infection has been linked to inflammation-related disease processes in the human host, including vascular diseases and chronic transplant rejection. The mechanisms through which CMV affects the pathogenesis of these diseases are for the most part unknown. To study the contributing role of the host immune response to CMV in these chronic inflammatory processes, we examined endothelial cell interactions with peripheral blood mononuclear cells (PBMC). Endothelial cultures were monitored for levels of fractalkine induction as a marker for initiating the host inflammatory response VSports手机版. Our results demonstrate that in the presence of CMV antigen PBMC from normal healthy CMV-seropositive donors produce soluble factors that induce fractalkine in endothelial cells. This was not observed in parallel assays with PBMC from seronegative donors. Examination of subset populations within the PBMC further revealed that CMV antigen-stimulated CD4(+) T cells were the source of the factors, gamma interferon and tumor necrosis factor alpha, driving fractalkine induction. Direct contact between CD4(+) cells and the endothelial monolayers is required for this fractalkine induction, where the endothelial cells appear to provide antigen presentation functions. These findings indicate that CMV may represent one member of a class of persistent pathogens where the antigen-specific T-cell response can result in the induction of fractalkine, leading to chronic inflammation and endothelial cell injury. .
Figures

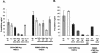
References
-
- Bettinotti, M. P., S. Solomon, M. Battiwala, N. Hensel, K. Ghazarian, D. Stroncek, and J. Barrett. 2003. T-cell response against the cytomegalovirus proteins pp65 and IE1. Hum. Immunol. 64:S73.
-
- Bitmanseur, A. D., S. L. Waldrop, C. J. Pitcher, E. Khatamzah, F. Kern, V. C. Maino, and L. J. Picker. 2001. Clonotypic structure of the human CD4+ memory T-cell response to CMV. J. Immunol. 167:1151-1163. - PubMed (VSports app下载)
-
- Bruning, J. H., M. C. Persoons, K. B. Lemstrom, F. S. Stals, E. De Clercq, and C. A. Bruggeman. 1994. Enhancement of transplantation-associated atherosclerosis by CMV, which can be prevented by antiviral therapy in the form of HPMPC. Transplant. Int. 7(Suppl. 1):S365-S370. - PubMed
-
- Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14and Toll-like receptor 2. J. Virol. 77:4588-4596. - V体育官网 - PMC - PubMed
Publication types
- Actions (V体育官网入口)
MeSH terms
- Actions (VSports注册入口)
- V体育安卓版 - Actions
- Actions (V体育ios版)
- Actions (VSports最新版本)
- "V体育官网" Actions
"VSports手机版" Substances
- "VSports最新版本" Actions
- V体育安卓版 - Actions
- "V体育安卓版" Actions
- VSports最新版本 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育ios版" Other Literature Sources
Research Materials
"VSports app下载" Miscellaneous

