"V体育ios版" Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response
- PMID: 15479734
- PMCID: PMC2172506
- DOI: 10.1083/jcb.200408003
Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response
Abstract
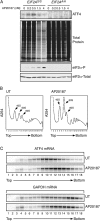
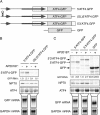
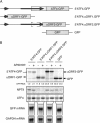
Stress-induced eukaryotic translation initiation factor 2 (eIF2) alpha phosphorylation paradoxically increases translation of the metazoan activating transcription factor 4 (ATF4), activating the integrated stress response (ISR), a pro-survival gene expression program. Previous studies implicated the 5' end of the ATF4 mRNA, with its two conserved upstream ORFs (uORFs), in this translational regulation. Here, we report on mutation analysis of the ATF4 mRNA which revealed that scanning ribosomes initiate translation efficiently at both uORFs and ribosomes that had translated uORF1 efficiently reinitiate translation at downstream AUGs. In unstressed cells, low levels of eIF2alpha phosphorylation favor early capacitation of such reinitiating ribosomes directing them to the inhibitory uORF2, which precludes subsequent translation of ATF4 and represses the ISR VSports手机版. In stressed cells high levels of eIF2alpha phosphorylation delays ribosome capacitation and favors reinitiation at ATF4 over the inhibitory uORF2. These features are common to regulated translation of GCN4 in yeast. The metazoan ISR thus resembles the yeast general control response both in its target genes and its mechanistic details. .
Figures


References
-
- Anthony, T.G., B.J. McDaniel, R.L. Byerley, B.C. McGrath, D.R. Cavener, M.A. McNurlan, and R.C. Wek. 2004. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem. 279:36553–36561. - PubMed (V体育官网入口)
-
- Chen, J.J., M.S. Throop, L. Gehrke, I. Kuo, J.K. Pal, M. Brodsky, and I.M. London. 1991. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc. Natl. Acad. Sci. USA. 88:7729–7733. - "V体育ios版" PMC - PubMed
-
- Dever, T.E. 2002. Gene-specific regulation by general translation factors. Cell. 108:545–556. - VSports最新版本 - PubMed
Publication types
"V体育安卓版" MeSH terms
- "VSports手机版" Actions
- Actions (VSports app下载)
- V体育官网入口 - Actions
- V体育官网入口 - Actions
- VSports在线直播 - Actions
- V体育ios版 - Actions
- "V体育2025版" Actions
- VSports最新版本 - Actions
- "V体育2025版" Actions
Substances
- Actions (VSports)
VSports app下载 - Grants and funding
"VSports在线直播" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports - Research Materials

