Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis
- PMID: 15337790
- PMCID: PMC2212736
- DOI: 10.1084/jem.20040762
V体育安卓版 - Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis
Abstract
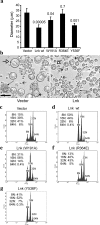
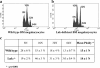
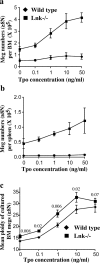
Thrombopoietin (Tpo) is the primary cytokine regulating megakaryocyte development and platelet production. Tpo signaling through its receptor, c-mpl, activates multiple pathways including signal transducer and activator of transcription (STAT)3, STAT5, phosphoinositide 3-kinase-Akt, and p42/44 mitogen-activated protein kinase (MAPK). The adaptor protein Lnk is implicated in cytokine receptor and immunoreceptor signaling. Here, we show that Lnk overexpression negatively regulates Tpo-mediated cell proliferation and endomitosis in hematopoietic cell lines and primary hematopoietic cells. Lnk attenuates Tpo-induced S-phase progression in 32D cells expressing mpl, and Lnk decreases Tpo-dependent megakaryocyte growth in bone marrow (BM)-derived megakaryocyte culture. Consistent with this result, we found that in both BM and spleen, Lnk-deficient mice exhibited increased numbers of megakaryocytes with increased ploidy compared with wild-type mice VSports手机版. In addition, Lnk-deficient megakaryocytes derived from BM and spleen showed enhanced sensitivity to Tpo during culture. The absence of Lnk caused enhanced and prolonged Tpo induction of STAT3, STAT5, Akt, and MAPK signaling pathways in CD41+ megakaryocytes. Furthermore, the Src homology 2 domain of Lnk is essential for Lnk's inhibitory function. In contrast, the conserved tyrosine near the COOH terminus is dispensable and the pleckstrin homology domain of Lnk contributes to, but is not essential for, inhibiting Tpo-dependent 32D cell growth or megakaryocyte development. Thus, Lnk negatively modulates mpl signaling pathways and is important for Tpo-mediated megakaryocytopoiesis in vivo. .
Figures


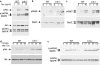
References
-
- Kaushansky, K. 1995. Thrombopoietin: the primary regulator of platelet production. Blood. 86:419–431. - PubMed
-
- Kaushansky, K. 2003. Thrombopoietin: a tool for understanding thrombopoiesis. J. Thromb. Haemost. 1:1587–1592. - "VSports最新版本" PubMed
-
- Gurney, A.L., K. Carver-Moore, F.J. de Sauvage, and M.W. Moore. 1994. Thrombocytopenia in c-mpl-deficient mice. Science. 265:1445–1447. - PubMed
-
- Alexander, W.S., A.W. Roberts, N.A. Nicola, R. Li, and D. Metcalf. 1996. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 87:2162–2170. - PubMed
Publication types
MeSH terms
- V体育安卓版 - Actions
- Actions (VSports手机版)
- Actions (V体育安卓版)
- Actions (V体育官网)
- "V体育ios版" Actions
- Actions (VSports手机版)
- VSports在线直播 - Actions
- Actions (VSports最新版本)
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- Actions (V体育ios版)
- "VSports app下载" Actions
- VSports - Actions
- Actions (V体育官网)
- Actions (V体育官网)
- V体育官网 - Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- "V体育官网入口" Actions
Substances
- VSports最新版本 - Actions
- V体育平台登录 - Actions
- Actions (V体育2025版)
- VSports在线直播 - Actions
- Actions (VSports app下载)
- VSports手机版 - Actions
- VSports - Actions
- Actions (V体育官网)
- Actions (VSports app下载)
- Actions (VSports最新版本)
- Actions (V体育安卓版)
- Actions (VSports手机版)
- "VSports最新版本" Actions
- VSports在线直播 - Actions
Grants and funding
LinkOut - more resources (VSports app下载)
Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育官网)
Miscellaneous

