Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition
- PMID: 15314173
- PMCID: PMC506971
- DOI: 10.1128/MCB.24.17.7654-7668.2004
Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition
Abstract
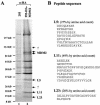
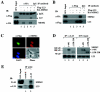
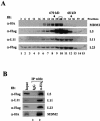
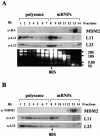
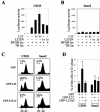
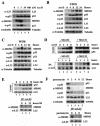
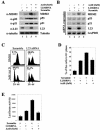
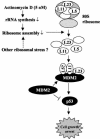
The p53-MDM2 feedback loop is vital for cell growth control and is subjected to multiple regulations in response to various stress signals VSports手机版. Here we report another regulator of this loop. Using an immunoaffinity method, we purified an MDM2-associated protein complex that contains the ribosomal protein L23. L23 interacted with MDM2, forming a complex independent of the 80S ribosome and polysome. The interaction of L23 with MDM2 was enhanced by treatment with actinomycin D but not by gamma-irradiation, leading to p53 activation. This activation was inhibited by small interfering RNA against L23. Ectopic expression of L23 reduced MDM2-mediated p53 ubiquitination and also induced p53 activity and G(1) arrest in p53-proficient U2OS cells but not in p53-deficient Saos-2 cells. These results reveal that L23 is another regulator of the p53-MDM2 feedback regulation. .
Copyright 2004 American Society for Microbiology
Figures


References
-
- Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. - PubMed
-
- Boyd, M. T., N. Vlatkovic, and D. S. Haines. 2000. A novel cellular protein (MTBP) binds to MDM2 and induces a G1 arrest that is suppressed by MDM2. J. Biol. Chem. 275:31883-31890. - PubMed
Publication types (VSports手机版)
- V体育安卓版 - Actions
MeSH terms
- "VSports app下载" Actions
- Actions (VSports最新版本)
- "VSports最新版本" Actions
- VSports注册入口 - Actions
- "VSports" Actions
- V体育2025版 - Actions
- "V体育ios版" Actions
- VSports最新版本 - Actions
- VSports注册入口 - Actions
Substances
- "V体育2025版" Actions
- "V体育官网入口" Actions
- VSports在线直播 - Actions
- Actions (VSports在线直播)
- "VSports手机版" Actions
Grants and funding (VSports在线直播)
V体育安卓版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
