Reactive nitrogen species-induced cell death requires Fas-dependent activation of c-Jun N-terminal kinase
- PMID: 15254243
- PMCID: PMC444859 (VSports手机版)
- DOI: 10.1128/MCB.24.15.6763-6772.2004
Reactive nitrogen species-induced cell death requires Fas-dependent activation of c-Jun N-terminal kinase
Abstract
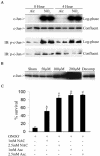
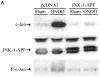
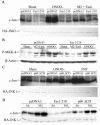
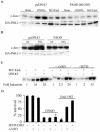
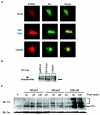
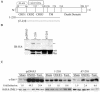
Nitrogen dioxide is a highly toxic reactive nitrogen species (RNS) recently discovered as an inflammatory oxidant with great potential to damage tissues. We demonstrate here that cell death by RNS was caused by c-Jun N-terminal kinase (JNK). Activation of JNK by RNS was density dependent and caused mitochondrial depolarization and nuclear condensation. JNK activation by RNS was abolished in cells lacking functional Fas or following expression of a truncated version of Fas lacking the intracellular death domain VSports手机版. In contrast, RNS induced JNK potently in cells expressing a truncated version of tumor necrosis factor receptor 1 or cells lacking tumor necrosis factor receptor 1 (TNF-R1), illustrating a dependence of Fas but not TNF-R1 in RNS-induced signaling to JNK. Furthermore, Fas was oxidized, redistributed, and colocalized with Fas-associated death domain (FADD) in RNS-exposed cells, illustrating that RNS directly targeted Fas. JNK activation and cell death by RNS occurred in a Fas ligand- and caspase-independent manner. While the activation of JNK by RNS or FasL required FADD, the cysteine-rich domain 1 containing preligand assembly domain required for FasL signaling was not involved in JNK activation by RNS. These findings illustrate that RNS cause cell death in a Fas- and JNK-dependent manner and that this occurs through a pathway distinct from FasL. Thus, avenues aimed at preventing the interaction of RNS with Fas may attenuate tissue damage characteristic of chronic inflammatory diseases that are accompanied by high levels of RNS. .
Figures






References
-
- Augusto, O., M. G. Bonini, A. M. Amanso, E. Linares, C. C. Santos, and S. L. De Menezes. 2002. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic. Biol. Med. 32:841-859. - PubMed
-
- Behrens, A., M. Sibilia, and E. F. Wagner. 1999. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21:326-329. - PubMed
-
- Brennan, M. L., W. Wu, X. Fu, Z. Shen, W. Song, H. Frost, C. Vadseth, L. Narine, E. Lenkiewicz, M. T. Borchers, A. J. Lusis, J. J. Lee, N. A. Lee, H. M. Abu-Soud, H. Ischiropoulos, and S. L. Hazen. 2002. A Tale of Two Controversies. Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 277:17415-17427. - PubMed
-
- Butterfield, L., B. Storey, L. Maas, and L. E. Heasley. 1997. c-Jun NH2-terminal kinase regulation of the apoptotic response of small cell lung cancer cells to ultraviolet radiation. J. Biol. Chem. 272:10110-10116. - PubMed (V体育2025版)
VSports最新版本 - Publication types
V体育平台登录 - MeSH terms
- "V体育2025版" Actions
- V体育安卓版 - Actions
- Actions (VSports)
- Actions (VSports)
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- "V体育平台登录" Actions
- Actions (VSports app下载)
- V体育2025版 - Actions
- "VSports手机版" Actions
- Actions (VSports)
- VSports - Actions
- Actions (VSports在线直播)
- V体育2025版 - Actions
- Actions (V体育平台登录)
- Actions (VSports在线直播)
- Actions (V体育ios版)
- VSports - Actions
- "V体育安卓版" Actions
- "V体育安卓版" Actions
Substances
- Actions (VSports最新版本)
- VSports手机版 - Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- V体育官网 - Actions
- V体育2025版 - Actions
- VSports最新版本 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
"VSports最新版本" Miscellaneous
