The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase (V体育安卓版)
- PMID: 14766977
- PMCID: PMC357064
- DOI: 10.1073/pnas.0307308101 (V体育安卓版)
The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase
Abstract
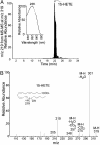
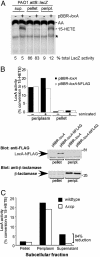
In mammals, lipoxygenases play key roles in inflammation by initiating the transformation of arachidonic acid into potent bioactive lipid mediators such as leukotrienes and lipoxins. In general, most bacteria are believed to lack lipoxygenases and their polyunsaturated fatty acid substrates. It is therefore of interest that an ORF (PA1169) with high homology to eukaryotic lipoxygenases was discovered by analysis of the whole-genome sequence of the opportunistic bacterial pathogen Pseudomonas aeruginosa. Using TLC and liquid chromatography-UV-tandem mass spectrometry (LC-UV-MS-MS), we demonstrate that PA1169 encodes a bacterial lipoxygenase (LoxA) that converts arachidonic acid into 15-hydroxyeicosatetraenoic acid (15-HETE). Although mammalian lipoxygenases are cytoplasmic enzymes, P VSports手机版. aeruginosa LoxA activity is secreted. Taken together, these results suggest a mechanism by which a pathogen-secreted lipoxygenase may modulate host defense and inflammation via alteration of the biosynthesis of local chemical mediators. .
Figures


V体育平台登录 - References
-
- Pollack, M. (2000) in Principles and Practice of Infectious Diseases, eds. Mandell, G. L., Bennet, J. E. & Dolin, R. (Churchill Livingstone, Philadelphia), Vol. 2, pp. 2310-2335.
-
- Samuelsson, B., Dahlen, S. E., Lindgren, J. A., Rouzer, C. A. & Serhan, C. N. (1987) Science 237, 1171-1176. - PubMed
-
- Rowley, A. F., Kühn, H. & Schewe, T., eds. (1998) Eicosanoids and Related Compounds in Plants and Animals (Portland Press, London).
-
- McMahon, B., Mitchell, S., Brady, H. R. & Godson, C. (2001) Trends Pharmacol. Sci. 22, 391-395. - V体育ios版 - PubMed
Publication types
V体育2025版 - MeSH terms
- "VSports app下载" Actions
- Actions (VSports注册入口)
- V体育ios版 - Actions
- "V体育官网" Actions
- Actions (VSports在线直播)
- "VSports" Actions
- "VSports手机版" Actions
- "VSports" Actions
Substances
- Actions (VSports手机版)
V体育官网入口 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

