Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils
- PMID: 12960399
- PMCID: "VSports注册入口" PMC196908
- DOI: 10.1073/pnas.1833375100 (V体育官网)
Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils (VSports注册入口)
Abstract
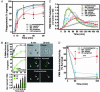
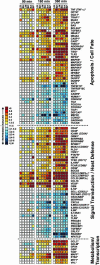
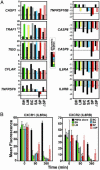
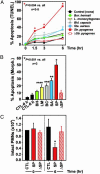
Human polymorphonuclear leukocytes (PMNs or neutrophils) are essential to the innate immune response against bacterial pathogens. Recent evidence suggests that PMN apoptosis facilitates resolution of inflammation during bacterial infection. Although progress has been made toward understanding apoptosis in neutrophils, very little is known about transcriptional regulation of this process during bacterial infection. To gain insight into the molecular processes that facilitate resolution of infection, we measured global changes in PMN gene expression during phagocytosis of a diverse group of bacterial pathogens. Genes encoding key effectors of apoptosis were up-regulated, and receptors critical to innate immune function were down-regulated during apoptosis induced by phagocytosis of Burkholderia cepacia, Borrelia hermsii, Listeria monocytogenes, Staphylococcus aureus, and Streptococcus pyogenes. Importantly, we identified genes that comprise a common apoptosis differentiation program in human PMNs after phagocytosis of pathogenic bacteria. Unexpectedly, phagocytosis of Str. pyogenes induced changes in neutrophil gene expression not observed with other pathogens tested, including down-regulation of 21 genes involved in responses to IFN. Compared with other bacteria, PMN apoptosis was significantly accelerated by Str. pyogenes and was followed by necrosis VSports手机版. Thus, we hypothesize that there are two fundamental outcomes for the interaction of bacterial pathogens with neutrophils: (i) phagocytosis of bacteria induces an apoptosis differentiation program in human PMNs that contributes to resolution of bacterial infection, or (ii) phagocytosis of microorganisms such as Str. pyogenes alters the apoptosis differentiation program in neutrophils, resulting in pathogen survival and disease. .
Figures

"V体育平台登录" References
-
- Kaplanski, G., Marin, V., Montero-Julian, F., Mantovani, A. & Farnarier, C. (2003) Trends Immunol. 24, 25–29. - PubMed
-
- Nauseef, W. M. & Clark, R. A. (2000) in Basic Principles in the Diagnosis and Management of Infectious Diseases, eds. Mandel, G. L., Bennett, J. E. & Dolin, R. (Churchill Livingstone, New York), Vol. 1, pp. 89–112.
-
- Lekstrom-Himes, J. A. & Gallin, J. I. (2000) N. Engl. J. Med. 343, 1703–1714. - PubMed
-
- Nathan, C. (2002) Nature 420, 846–852. - PubMed (VSports)
-
- Scaife, H., Woldehiwet, Z., Hart, C. A. & Edwards, S. W. (2003) Infect. Immun. 71, 1995–2001. - V体育官网入口 - PMC - PubMed
MeSH terms
- Actions (V体育ios版)
- V体育官网 - Actions
- Actions (V体育官网入口)
V体育ios版 - LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育官网入口)

