VSports在线直播 - Targeted disruption of p185/Cul7 gene results in abnormal vascular morphogenesis
- PMID: 12904573
- PMCID: PMC187864 (V体育官网)
- DOI: 10.1073/pnas.1733908100
Targeted disruption of p185/Cul7 gene results in abnormal vascular morphogenesis (V体育ios版)
Abstract
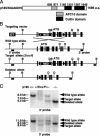
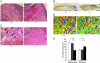
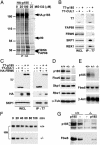
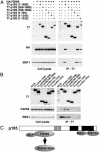
Cul1, a member of the cullin ubiquitin ligase family, forms a multiprotein complex known as SCF and plays an essential role in numerous cellular and biological activities. A Cul1 homologue, p185 (Cul7), has been isolated as an simian virus 40 large T antigen-binding protein. To understand the physiological role of p185, we generated mice lacking p185 VSports手机版. p185-/- embryos are runted and die immediately after birth because of respiratory distress. Dermal and hypodermal hemorrhage is detected in mutant embryos at late gestational stage. p185-/- placentas show defects in the differentiation of the trophoblast lineage with an abnormal vascular structure. We demonstrate that p185 forms an SCF-like complex with Skp1, Rbx1, Fbw6 (Fbx29), and FAP68 (FAP48, glomulin). FAP68 has recently been identified as a gene responsible for familial glomuvenous malformation. These results suggest that p185 forms a multiprotein complex and plays an important role in vascular morphogenesis. .
Figures

"V体育官网" References
-
- Hershko, A. & Ciechanover, A. (1998) Annu. Rev. Biochem. 67, 425–479. - PubMed
-
- Deshaies, R. J. (1999) Annu. Rev. Cell Dev. Biol. 15, 435–467. - PubMed (V体育2025版)
-
- Zhang, H., Kobayashi, R., Galaktionov, K. & Beach, D. (1995) Cell 82, 915–925. - PubMed
-
- Koepp, D. M., Harper, J. W. & Elledge, S. J. (1999) Cell 97, 431–434. - "V体育ios版" PubMed
-
- Bodine, S. C., Latres, E., Baumhueter, S., Lai, V. K., Nunez, L., Clarke, B. A., Poueymirou, W. T., Panaro, F. J., Na, E., Dharmarajan, K., et al. (2001) Science 294, 1704–1708. - PubMed
Publication types
MeSH terms
- "V体育官网入口" Actions
- VSports最新版本 - Actions
- V体育官网入口 - Actions
- "VSports app下载" Actions
- Actions (V体育官网入口)
- Actions (V体育2025版)
- Actions (V体育官网入口)
- "VSports手机版" Actions
- "VSports注册入口" Actions
- "V体育官网" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- "VSports" Actions
- Actions (VSports app下载)
- V体育官网 - Actions
Substances
- "V体育官网" Actions
- Actions (VSports)
- V体育安卓版 - Actions
- "V体育ios版" Actions
V体育ios版 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育2025版 - Molecular Biology Databases
Research Materials

