"V体育2025版" Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development
- PMID: 12810684
- PMCID: PMC2193952
- DOI: 10.1084/jem.20022234
VSports在线直播 - Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development
Abstract
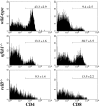
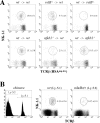
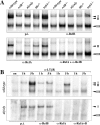
Natural killer T (NKT) cells have been implicated in diverse immune responses ranging from suppression of autoimmunity to tumor rejection. Thymus-dependent NKT cells are positively selected by the major histocompatibility complex class I-like molecule CD1d, but the molecular events downstream of CD1d are still poorly understood. Here, we show that distinct members of the Rel/nuclear factor (NF)-kappa B family of transcription factors were required in both hematopoietic and nonhematopoietic cells for normal development of thymic NKT cells. Activation of NF-kappa B via the classical I kappa B alpha-regulated pathway was required in a cell autonomous manner for the transition of NK-1. 1-negative precursors that express the TCR V alpha 14-J alpha 18 chain to mature NK-1. 1-positive NKT cells. The Rel/NF-kappa B family member RelB, on the other hand, had to be expressed in radiation resistant thymic stromal cells for the generation of early NK-1. 1-negative NKT precursors VSports手机版. Moreover, NF-kappa B-inducing kinase (NIK) was required for both constitutive thymic DNA binding of RelB and the specific induction of RelB complexes in vitro. Thus, distinct Rel/NF-kappa B family members in hematopoietic and nonhematopoietic cells regulate NKT cell development with a unique requirement for NIK-mediated activation of RelB in thymic stroma. .
VSports注册入口 - Figures

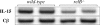


References
-
- Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. - V体育平台登录 - PubMed
-
- Godfrey, D.I., K.J. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. - V体育ios版 - PubMed
-
- MacDonald, H.R. 2002. Development and selection of NKT cells. Curr. Opin. Immunol. 14:250–254. - V体育官网入口 - PubMed
-
- Bendelac, A., O. Lantz, M.E. Quimby, J.W. Yewdell, J.R. Bennink, and R.R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science. 268:863–865. - PubMed
Publication types
"VSports最新版本" MeSH terms
- V体育2025版 - Actions
- Actions (VSports)
- V体育官网 - Actions
- "V体育ios版" Actions
- Actions (V体育官网)
- "V体育ios版" Actions
- "V体育官网入口" Actions
- "VSports" Actions
- "VSports手机版" Actions
- "VSports" Actions
- V体育ios版 - Actions
- "V体育安卓版" Actions
Substances
- Actions (V体育ios版)
- Actions (V体育2025版)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
"VSports手机版" Miscellaneous

