Ras promotes p21(Waf1/Cip1) protein stability via a cyclin D1-imposed block in proteasome-mediated degradation
- PMID: 12727871
- PMCID: PMC156064
- DOI: 10.1093/emboj/cdg189
VSports手机版 - Ras promotes p21(Waf1/Cip1) protein stability via a cyclin D1-imposed block in proteasome-mediated degradation
Abstract
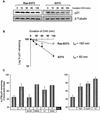
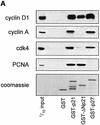
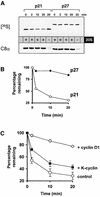
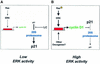
Ras promotes the accumulation of the cyclin-dependent kinase inhibitor p21(Waf1/Cip1) (p21) VSports手机版. Previous studies reported that acute Raf/MEK/ERK activation elevates p21 protein levels by increased transcription. However, we have found that p21 induction in Ras-transformed murine fibroblasts occurs principally by a post-translational mechanism. Chronic activation of the Raf/MEK/ERK pathway blocked proteasome-mediated p21 degradation, resulting in accumulation of p21 protein with an elevated half-life. The stabilization of p21 by Ras was accompanied by high levels of p21-associated cyclin D1 and, similarly to Ras, cyclin D1 was sufficient to inhibit the proteasome-mediated p21 degradation. Knock-down of cyclin D1 by RNA interference confirmed that Ras-induced p21 stabilization was dependent upon cyclin D1 expression. We show that p21 directly binds to the C8alpha subunit of the 20S proteasome complex and that by competing for binding, cyclin D1 inhibits p21 degradation by purified 20S complexes in vitro. Therefore, we propose that Ras stabilizes p21 by promoting the formation of p21-cyclin D1 complexes that prevent p21 association with, and subsequent degradation by, the 20S proteasome. .
Figures






References (V体育平台登录)
-
- Adams P.D., Sellers,W.R., Sharma,S.K., Wu,A.D., Nalin,C.M. and Kaelin,W.G.,Jr (1996) Identification of a cyclin–cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol., 16, 6623–6633. - V体育安卓版 - PMC - PubMed
-
- Adnane J., Bizouarn,F.A., Qian,Y., Hamilton,A.D. and Sebti,S.M. (1998) p21WAF1/CIP1 is upregulated by the geranylgeranyltransferase I inhibitor GGTI-298 through a transforming growth factor β- and Sp1-responsive element: involvement of the small GTPase rhoA. Mol. Cell. Biol., 18, 6962–6970. - PMC (VSports最新版本) - PubMed
-
- Alessandrini A., Chiaur,D.S. and Pagano,M. (1997) Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia, 11, 342–345. - PubMed
-
- Alt J.R., Gladden,A.B. and Diehl,J.A. (2002) p21Cip1 promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem., 277, 8517–8523. - PubMed
-
- Ball K.L., Lain,S., Fahraeus,R., Smythe,C. and Lane,D.P. (1997) Cell-cycle arrest and inhibition of Cdk4 activity by small peptides based on the carboxy-terminal domain of p21WAF1. Curr. Biol., 7, 71–80. - PubMed
Publication types
MeSH terms
- "VSports app下载" Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- Actions (VSports手机版)
- "V体育2025版" Actions
- VSports手机版 - Actions
- V体育安卓版 - Actions
Substances
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

