VSports手机版 - Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation
- PMID: 12677000
- PMCID: "VSports手机版" PMC153564
- DOI: 10.1073/pnas.0430973100
Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation
Abstract
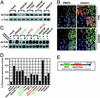
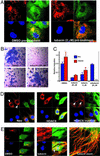
Protein acetylation, especially histone acetylation, is the subject of both research and clinical investigation. At least four small-molecule histone deacetylase inhibitors are currently in clinical trials for the treatment of cancer. These and other inhibitors also affect microtubule acetylation VSports手机版. A multidimensional, chemical genetic screen of 7,392 small molecules was used to discover "tubacin," which inhibits alpha-tubulin deacetylation in mammalian cells. Tubacin does not affect the level of histone acetylation, gene-expression patterns, or cell-cycle progression. We provide evidence that class II histone deacetylase 6 (HDAC6) is the intracellular target of tubacin. Only one of the two catalytic domains of HDAC6 possesses tubulin deacetylase activity, and only this domain is bound by tubacin. Tubacin treatment did not affect the stability of microtubules but did decrease cell motility. HDAC6 overexpression disrupted the localization of p58, a protein that mediates binding of Golgi elements to microtubules. Our results highlight the role of alpha-tubulin acetylation in mediating the localization of microtubule-associated proteins. They also suggest that small molecules that selectively inhibit HDAC6-mediated alpha-tubulin deacetylation, a first example of which is tubacin, might have therapeutic applications as antimetastatic and antiangiogenic agents. .
"V体育2025版" Figures


References
-
- Grozinger C M, Schreiber S L. Chem Biol. 2002;9:3–16. - PubMed
-
- Khochbin S, Verdel A, Lemercier C, Seigneurin-Berny D. Curr Opin Genet Dev. 2001;11:162–166. - PubMed (VSports手机版)
-
- Yoshida M, Kijima M, Akita M, Beppu T. J Biol Chem. 1990;265:17174–17179. - PubMed
-
- Kijima M, Yoshida M, Sugita K, Horinouchi S, Beppu T. J Biol Chem. 1993;268:22429–22435. - PubMed (VSports)
-
- Taunton J, Hassig C A, Schreiber S L. Science. 1996;272:408–411. - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- VSports注册入口 - Actions
- "V体育ios版" Actions
- "V体育官网" Actions
- VSports app下载 - Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- "VSports手机版" Actions
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- V体育官网入口 - Actions
- "V体育安卓版" Actions
- Actions (VSports注册入口)
- "V体育安卓版" Actions
- "V体育安卓版" Actions
V体育平台登录 - Substances
- V体育官网入口 - Actions
- "VSports在线直播" Actions
- Actions (V体育平台登录)
- V体育平台登录 - Actions
- "VSports在线直播" Actions
LinkOut - more resources
"VSports最新版本" Full Text Sources
Other Literature Sources
Molecular Biology Databases

