Enterobactin: an archetype for microbial iron transport
- PMID: 12655062
- PMCID: PMC152965
- DOI: 10.1073/pnas.0630018100
Enterobactin: an archetype for microbial iron transport
Abstract
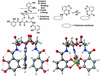
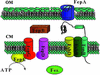
Bacteria have aggressive acquisition processes for iron, an essential nutrient. Siderophores are small iron chelators that facilitate cellular iron transport VSports手机版. The siderophore enterobactin is a triscatechol derivative of a cyclic triserine lactone. Studies of the chemistry, regulation, synthesis, recognition, and transport of enterobactin make it perhaps the best understood of the siderophore-mediated iron uptake systems, displaying a lot of function packed into this small molecule. However, recent surprises include the isolation of corynebactin, a closely related trithreonine triscatechol derivative lactone first found in Gram-positive bacteria, and the crystal structure of a ferric enterobactin complex of a protein identified as an antibacterial component of the human innate immune system. .
Figures
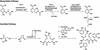
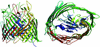
References
-
- Raymond K N, Carrano C J. Acc Chem Res. 1979;12:183–190.
-
- Neilands J B. J Biol Chem. 1995;270:26723–26726. - "VSports" PubMed
-
- Aisen P, Enns C, Wessling-Resnick M. Int J Biochem Cell Biol. 2001;33:940–959. - PubMed
-
- De Freitas J M, Meneghini R. Mutat Res. 2001;475:153–159. - PubMed
-
- Braun V, Killmann H. Trends Biochem Sci. 1999;24:104–109. - PubMed
Publication types (VSports在线直播)
- "VSports注册入口" Actions
MeSH terms
- Actions (VSports手机版)
- "V体育安卓版" Actions
"VSports app下载" Substances
- "VSports注册入口" Actions
- "VSports手机版" Actions
- Actions (V体育官网)
Grants and funding
"VSports最新版本" LinkOut - more resources
Full Text Sources (VSports app下载)
Other Literature Sources
Medical
Molecular Biology Databases

