V体育官网入口 - Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins
- PMID: 12654791
- PMCID: PMC152057
- DOI: 10.1128/IAI.71.4.1774-1783.2003
Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins
Abstract
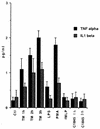
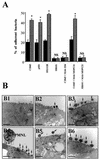
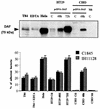
Ulcerative colitis and Crohn's disease are inflammatory bowel diseases thought to involve strains of Escherichia coli VSports手机版. We report here that two wild-type Afa/Dr diffusely adhering E. coli (DAEC) strains, C1845 and IH11128, which harbor the fimbrial F1845 adhesin and the Dr hemagglutinin, respectively, and the E. coli laboratory strain HB101, transformed with the pSSS1 plasmid to produce Afa/Dr F1845 adhesin, all induced interleukin-8 (IL-8) production and transepithelial migration of polymorphonuclear leukocytes (PMNL) in polarized monolayers of the human intestinal cell line T84 grown on semipermeable filters. We observed that after PMNL migration, expression of decay-accelerating factor (DAF, or CD55), the brush border-associated receptor for Afa/Dr adhesins, was strongly enhanced, increasing the adhesion of Afa/Dr DAEC bacteria. When examining the mechanism by which DAF expression was enhanced, we observed that the PMNL transepithelial migration induced epithelial synthesis of tumor necrosis factor alpha and IL-1beta, which in turn promoted the upregulation of DAF. .
V体育官网 - Figures


References
-
- Abrami, L., M. Fivaz, T. Kobayashi, T. Kinoshita, R. G. Parton, and F. G. van der Goot. 2001. Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J. Biol. Chem. 276:30729-30736. - "VSports注册入口" PubMed
-
- Andoh, A., Y. Fujiyama, K. Sumiyoshi, H. Sakumoto, and T. Bamba. 1996. Interleukin 4 acts as an inducer of decay-accelerating factor gene expression in human intestinal epithelial cells. Gastroenterology 111:911-918. - PubMed
-
- Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 4:635-648. - PubMed
-
- Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut 38:248-253. - "V体育ios版" PMC - PubMed
MeSH terms
- Actions (VSports app下载)
- Actions (V体育官网入口)
- Actions (V体育官网)
- "V体育官网" Actions
- V体育官网 - Actions
- V体育官网 - Actions
- Actions (V体育ios版)
- "VSports最新版本" Actions
- Actions (VSports app下载)
- "V体育官网入口" Actions
- V体育官网 - Actions
- Actions (V体育官网入口)
- Actions (V体育平台登录)
Substances
- VSports注册入口 - Actions
LinkOut - more resources
"VSports app下载" Full Text Sources
"VSports" Miscellaneous

