A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I
- PMID: 12511593
- PMCID: PMC151839
- DOI: 10.1172/JCI16808
A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I
V体育2025版 - Abstract
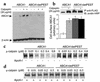
Cholesterol-loaded macrophage foam cells are a central component of atherosclerotic lesions. ABCA1, the defective molecule in Tangier disease, mediates the efflux of phospholipids and cholesterol from cells to apoA-I, reversing foam cell formation. In ABCA1, we identified a sequence rich in proline, glutamic acid, serine, and threonine (PEST sequence) that enhances the degradation of ABCA1 by calpain protease and thereby controls the cell surface concentration and cholesterol efflux activity of ABCA1. In an apparent positive feedback loop, apoA-I binds ABCA1, promotes lipid efflux, inhibits calpain degradation, and leads to increased levels of ABCA1. ApoA-I infusion also increases ABCA1 in vivo. These studies reveal a novel mode of regulation of ABCA1 by PEST sequence-mediated calpain proteolysis that appears to be reversed by apolipoprotein-mediated phospholipid efflux. Inhibition of ABCA1 degradation by calpain could represent a novel therapeutic approach to increasing macrophage cholesterol efflux and decreasing atherosclerosis. VSports手机版.
Figures (V体育官网)



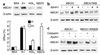


VSports - References
-
- Brown MS, Ho YK, Goldstein JL. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J. Biol. Chem. 1980;255:9344–9352. - V体育2025版 - PubMed
-
- Ho YK, Brown MS, Goldstein JL. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J. Lipid Res. 1980;21:391–398. - PubMed
-
- Oram JF, Albers JJ, Cheung MC, Bierman EL. The effects of subfractions of high density lipoprotein on cholesterol efflux from cultured fibroblasts. Regulation of low density lipoprotein receptor activity. J. Biol. Chem. 1981;256:8348–8356. - PubMed
-
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. - "V体育2025版" PubMed
Publication types
- "V体育官网" Actions
"VSports注册入口" MeSH terms
- "V体育ios版" Actions
- Actions (V体育官网入口)
- Actions (V体育官网入口)
- VSports app下载 - Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- VSports注册入口 - Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- Actions (V体育安卓版)
Substances
- V体育官网 - Actions
- Actions (V体育2025版)
- "VSports手机版" Actions
- "VSports手机版" Actions
- V体育官网入口 - Actions
- "VSports app下载" Actions
- VSports注册入口 - Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育官网" Other Literature Sources
"VSports注册入口" Molecular Biology Databases

