Antimicrobial peptides from human platelets
- PMID: 12438321
- PMCID: PMC132966
- DOI: "V体育官网入口" 10.1128/IAI.70.12.6524-6533.2002
"V体育安卓版" Antimicrobial peptides from human platelets
Abstract
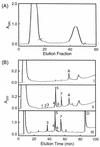
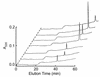
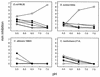
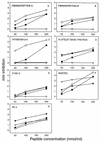
Platelets share structural and functional similarities with granulocytes known to participate in antimicrobial host defense. To evaluate the potential antimicrobial activities of platelet proteins, normal human platelets were stimulated with human thrombin in vitro. Components of the stimulated-platelet supernatants were purified to homogeneity by reversed-phase high-performance liquid chromatography. Purified peptides with inhibitory activity against Escherichia coli ML35 in an agar diffusion antimicrobial assay were characterized by mass spectrometry, amino acid analysis, and sequence determination. These analyses enabled the identification of seven thrombin-releasable antimicrobial peptides from human platelets: platelet factor 4 (PF-4), RANTES, connective tissue activating peptide 3 (CTAP-3), platelet basic protein, thymosin beta-4 (Tbeta-4), fibrinopeptide B (FP-B), and fibrinopeptide A (FP-A). With the exception of FP-A and FP-B, all peptides were also purified from acid extracts of nonstimulated platelets VSports手机版. The in vitro antimicrobial activities of the seven released peptides were further tested against bacteria (E. coli and Staphylococcus aureus) and fungi (Candida albicans and Cryptococcus neoformans). Each peptide exerted activity against at least two organisms. Generally, the peptides were more potent against bacteria than fungi, activity was greater at acidic pHs, and antimicrobial activities were dose dependent. Exceptions to these observations were observed with PF-4, which displayed a bimodal dose-response relationship in microbicidal assays, and Tbeta-4, which had greater activity at alkaline pHs. At concentrations at which they were individually sublethal, PF-4 and CTAP-3 exerted synergistic microbicidal activity against E. coli. Collectively, these findings suggest a direct antimicrobial role for platelets as they are activated to release peptides in response to trauma or mediators of inflammation. .
Figures


Comment in
-
Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense.Infect Immun. 2002 Dec;70(12):6515-7. doi: 10.1128/IAI.70.12.6515-6517.2002. Infect Immun. 2002. PMID: 12438319 Free PMC article. Review. No abstract available.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Bessalle, R., H. Haas, A. Goria, I. Shalit, and M. Fridkin. 1992. Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrob. Agents Chemother. 36:313-317. - V体育ios版 - PMC - PubMed
-
- Carney, D. H. 1992. Postclotting cellular effects of thrombin mediated by interaction with high-affinity thrombin receptors, p. 351-370. In L. J. Berliner (ed.), Thrombin: structure and function. Plenum Press, New York, N.Y.
-
- Carroll, S. F., and R. J. Martinez. 1981. Antibacterial peptide from normal rabbit serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry 20:5973-5981. - PubMed
-
- Chang, F. Y., N. Singh, T. Gayowski, M. M. Wagener, S. M. Mietzner, J. E. Stout, and I. G. Marino. 2000. Thrombocytopenia in liver transplant recipients: predictors, impact on fungal infections, and role of endogenous thrombopoietin. Transplantation 69:70-75. - PubMed (VSports在线直播)
Publication types
- Actions (VSports最新版本)
- Actions (V体育官网)
MeSH terms
- VSports app下载 - Actions
- "V体育2025版" Actions
- "V体育平台登录" Actions
- V体育官网入口 - Actions
- "VSports在线直播" Actions
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- "V体育ios版" Actions
- "VSports" Actions
- Actions (V体育官网入口)
"VSports" Substances
- V体育平台登录 - Actions
- V体育官网入口 - Actions
- Actions (VSports注册入口)
- VSports app下载 - Actions
VSports最新版本 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育2025版)
Medical
Miscellaneous

