TIS7 interacts with the mammalian SIN3 histone deacetylase complex in epithelial cells
- PMID: 12198164
- PMCID: "VSports在线直播" PMC125408
- DOI: 10.1093/emboj/cdf461
TIS7 interacts with the mammalian SIN3 histone deacetylase complex in epithelial cells
V体育安卓版 - Abstract
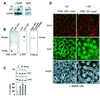
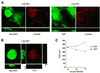
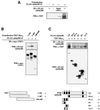
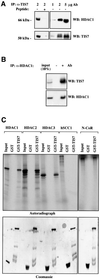
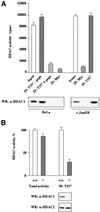
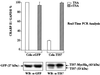
The mammalian SIN3 complex consists of histone deacetylases (HDAC1, HDAC2), several known proteins (SAP30, N-CoR) and as yet unidentified proteins. Here we show that the mouse tetradecanoyl phorbol acetate induced sequence 7 (TIS7) protein is a novel transcriptional co-repressor that can associate with the SIN3 complex VSports手机版. We have identified tis7 as a gene that is up-regulated upon loss of polarity in a mouse mammary gland epithelial cell line expressing an estrogen-inducible c-JunER fusion protein. In unpolarized cells, TIS7 protein levels increase and TIS7 translocates into the nucleus. Overexpression of tis7 causes loss of polarity and represses a set of genes, as revealed by cDNA microarray analysis. We have shown that TIS7 protein interacts with several proteins of the SIN3 complex (mSin3B, HDAC1, N-CoR and SAP30) by yeast two-hybrid screening and co-immunoprecipitations. TIS7 co-immunoprecipitated HDAC complex is enzymatically active and represses a GAL4-dependent reporter transcription. The transcriptional repression of endogenous genes by tis7 overexpression is HDAC dependent. Thus, we propose TIS7 as a transcriptional co-repressor affecting the expression of specific genes in a HDAC activity-dependent manner during cell fate decisions, e. g. scattering. .
Figures


References
-
- Ahringer J. (2000) NuRD and SIN3 histone deacetylase complexes in development. Trends Genet., 16, 351–356. - PubMed (V体育官网)
-
- Alkema M.J., Jacobs,J., Voncken,J.W., Jenkins,N.A., Copeland,N.G., Satijn,D.P., Otte,A.P., Berns,A. and van Lohuizen,M. (1997) MPc2, a new murine homolog of the Drosophila polycomb protein is a member of the mouse polycomb transcriptional repressor complex. J. Mol. Biol., 273, 993–1003. - PubMed
-
- Alland L., Muhle,R., Hou,H.,Jr, Potes,J., Chin,L., Schreiber-Agus,N. and DePinho,R.A. (1997) Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature, 387, 49–55. - PubMed
-
- Ayer D.E. (1999) Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol., 9, 193–198. - "V体育安卓版" PubMed
-
- Ayer D.E., Lawrence,Q.A. and Eisenman,R.N. (1995) Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell, 80, 767–776. - PubMed
Publication types
MeSH terms
- VSports - Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- "V体育官网入口" Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
- "VSports手机版" Actions
- "V体育ios版" Actions
- "VSports app下载" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- VSports - Actions
- "V体育官网入口" Actions
- V体育2025版 - Actions
Substances
- VSports - Actions
- Actions (V体育安卓版)
- "V体育官网" Actions
- "V体育ios版" Actions
- Actions (VSports最新版本)
- Actions (V体育平台登录)
- Actions (VSports在线直播)
- Actions (V体育2025版)
- VSports手机版 - Actions
- "VSports app下载" Actions
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

