Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens
- PMID: 12163561
- PMCID: PMC2193940
- DOI: 10.1084/jem.20011612
Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens
Abstract
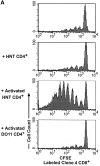
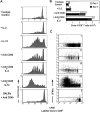
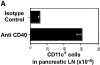
Professional antigen-presenting cells (APCs) are capable of transporting self-antigens from peripheral tissues to secondary lymphoid organs where they are presented to potentially autoreactive CD8(+) T cells. In the absence of an inflammatory response, this results in immune tolerance. The presence of activated, antigen-specific CD4(+) T cells converts this tolerogenic encounter into an immunogenic one by promoting extensive proliferation of CD8(+) T cells and their development into effectors. Surprisingly, activation of APCs with an agonistic antibody specific for CD40 could not substitute for CD4(+) help in this task. Anti-CD40 induced recruitment of dendritic cells expressing high levels of B7 costimulatory molecules into the lymph nodes, which in turn, greatly enhanced activation and expansion of CD8(+) T cells. However, these activated CD8(+) cells did not demonstrate effector function VSports手机版. We conclude that proliferative potential and gain of effector function are separable events in the differentiation program of CD8(+) T cells. .
Figures







References
-
- Stockinger, B. 1999. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv. Immunol. 71:229–265. - PubMed
-
- Heath, W.R., and F.R. Carbone. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47–64. - V体育官网 - PubMed
-
- Albert, M.L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. - PubMed
-
- Huang, F.P., N. Platt, M. Wykes, J.R. Major, T.J. Powell, C.D. Jenkins, and G.G. MacPherson. 2000. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191:435–444. - "VSports app下载" PMC - PubMed
-
- Kurts, C., M. Cannarile, I. Klebba, and T. Brocker. 2001. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 166:1439–1442. - PubMed
Publication types (VSports在线直播)
MeSH terms
- Actions (VSports app下载)
- VSports最新版本 - Actions
- V体育官网 - Actions
- "VSports app下载" Actions
- Actions (V体育安卓版)
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- V体育平台登录 - Actions
Substances
- "VSports注册入口" Actions
- Actions (V体育官网)
- Actions (VSports注册入口)
Grants and funding (V体育官网)
LinkOut - more resources
Full Text Sources
Research Materials (VSports注册入口)
"VSports" Miscellaneous

