Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells
- PMID: 12070280
- PMCID: PMC2193564
- DOI: 10.1084/jem.20020066
Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells
Abstract
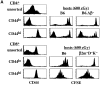
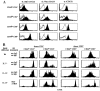
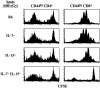
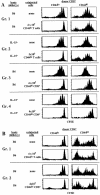
The overall size and composition of the pool of naive and memory T cells are tightly regulated by homeostatic mechanisms VSports手机版. Recent work has shown that homeostasis of naive T cells is controlled by two factors, self-major histocompatibility complex (MHC)/peptide ligands and a cytokine, interleukin (IL)-7. In particular, contact with these two factors is required for naive CD4+ and CD8+ cells to undergo "homeostatic" proliferation, i. e. , proliferation induced as a consequence of severe T cell depletion. In contrast to naive T cells, the factors that drive memory T cells to undergo homeostatic proliferation are poorly understood. To address this issue, purified memory phenotype CD4+ and CD8+ cells from normal mice were adoptively transferred into various gene-knockout mice rendered T cell-deficient by sublethal irradiation. Three findings are reported. First, unlike naive T cells, homeostatic proliferation of memory T cells is largely MHC independent. Second, memory CD8+ cells can utilize either IL-7 or IL-15 to undergo homeostatic proliferation; however, in the absence of both IL-7 and IL-15, homeostatic proliferation fails to occur. Third, unlike memory CD8+ cells, homeostatic proliferation of memory CD4+ cells is independent of IL-7 and IL-15 (also IL-4). Thus, the homeostatic proliferation mechanisms that control memory CD8+ cells and memory CD4+ cells are quite distinct. .
Figures


"VSports手机版" Comment in
-
Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15.J Exp Med. 2002 Jun 17;195(12):F49-52. doi: 10.1084/jem.20020767. J Exp Med. 2002. PMID: 12070294 Free PMC article. No abstract available.
References
-
- Freitas, A.A., and B.B. Rocha. 1993. Lymphocyte lifespan: homeostasis, selection and competition. Immunol. Today. 14:25–29. - PubMed
-
- Bell, E.B., and S.M. Sparshott. 1997. The peripheral T-cell pool: regulation by non-antigen induced proliferation? Sem. Immunol. 9:347–353. - PubMed
-
- Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. - PubMed
-
- Viret, C., F.S. Wong, and C.A. Janeway, Jr. 1999. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 10:559–568. - PubMed
"V体育官网" Publication types
"V体育2025版" MeSH terms
- "V体育官网" Actions
- "VSports app下载" Actions
- "V体育安卓版" Actions
- Actions (V体育ios版)
- V体育平台登录 - Actions
- Actions (VSports手机版)
Substances
- "V体育2025版" Actions
- Actions (VSports)
Grants and funding
- CA38355/CA/NCI NIH HHS/United States
- AI21487/AI/NIAID NIH HHS/United States
- AI46710/AI/NIAID NIH HHS/United States
- HL07196/HL/NHLBI NIH HHS/United States
- R01 AI046710/AI/NIAID NIH HHS/United States
- R37 CA038355/CA/NCI NIH HHS/United States (VSports在线直播)
- AG20186/AG/NIA NIH HHS/United States
- R01 AI045809/AI/NIAID NIH HHS/United States
- T32 AI007244/AI/NIAID NIH HHS/United States
- AI07244/AI/NIAID NIH HHS/United States
- VSports手机版 - R01 AG020186/AG/NIA NIH HHS/United States
- AI45809/AI/NIAID NIH HHS/United States
- AI41079/AI/NIAID NIH HHS/United States (V体育安卓版)
LinkOut - more resources
Full Text Sources
VSports app下载 - Other Literature Sources
Research Materials

