Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations
- PMID: 12045264
- PMCID: PMC150995 (VSports app下载)
- DOI: 10.1172/JCI14858 (V体育官网)
Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations
Abstract
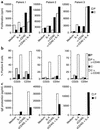
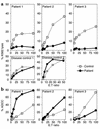
NF-kappaB essential modifier (NEMO), also known as IKK-gamma, is a member of the I-kappaB kinase complex responsible for phosphorylating I-kappaB, allowing the release and activation of NF-kappaB. Boys with an expressed NEMO mutation have an X-linked syndrome characterized by hypohidrotic ectodermal dysplasia with immune deficiency (HED-ID). The immunophenotype resulting from NEMO mutation is highly variable, with deficits in both T and B cell responses. We evaluated three patients with NEMO mutations (L153R, Q403X, and C417R) and HED-ID who had evidence of defective CD40 signaling. All three patients had normal percentages of peripheral blood NK cells, but impaired NK cell cytotoxic activity. This was not due to a generalized defect in cytotoxicity because antibody-dependent cellular cytotoxicity was intact. This abnormality was partially reversed by in vitro addition of IL-2, which was also able to induce NF-kappaB activation VSports手机版. In one patient with recurrent cytomegalovirus infections, administration of IL-2 partially corrected the NK cell killing deficit. These data suggest that NEMO participates in signaling pathways leading to NK cell cytotoxicity and that IL-2 can activate NF-kappaB and partially overcome the NK cell defect in patients with NEMO mutations. .
Figures (VSports)



References
-
- Kere J, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. - PubMed
-
- Monreal AW, et al. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet. 1999;22:366–369. - PubMed
-
- Headon DJ, et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414:913–916. - PubMed (VSports手机版)
-
- Abinun M, Spickett G, Appleton AL, Flood T, Cant AJ. Anhidrotic ectodermal dysplasia associated with specific antibody deficiency. Eur J Pediatr. 1996;155:146–147. - PubMed
Publication types
"VSports" MeSH terms
- Actions (VSports)
- VSports注册入口 - Actions
- "VSports" Actions
- Actions (VSports在线直播)
- V体育ios版 - Actions
- "V体育官网" Actions
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- Actions (VSports注册入口)
- "V体育官网入口" Actions
VSports在线直播 - Substances
- "VSports app下载" Actions
- "VSports在线直播" Actions
- Actions (V体育ios版)
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

