The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development
- PMID: 12000792
- PMCID: PMC186247
- DOI: 10.1101/gad.988402
The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development
Abstract
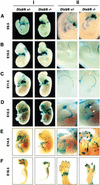
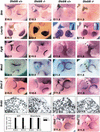
Dlx homeobox genes are mammalian homologs of the Drosophila Distal-less (Dll) gene. The Dlx/Dll gene family is of ancient origin and appears to play a role in appendage development in essentially all species in which it has been identified. In Drosophila, Dll is expressed in the distal portion of the developing appendages and is critical for the development of distal structures. In addition, human Dlx5 and Dlx6 homeobox genes have been identified as possible candidate genes for the autosomal dominant form of the split-hand/split-foot malformation (SHFM), a heterogeneous limb disorder characterized by missing central digits and claw-like distal extremities. Targeted inactivation of Dlx5 and Dlx6 genes in mice results in severe craniofacial, axial, and appendicular skeletal abnormalities, leading to perinatal lethality. For the first time, Dlx/Dll gene products are shown to be critical regulators of mammalian limb development, as combined loss-of-function mutations phenocopy SHFM. Furthermore, spatiotemporal-specific transgenic overexpression of Dlx5, in the apical ectodermal ridge of Dlx5/6 null mice can fully rescue Dlx/Dll function in limb outgrowth. VSports手机版.
V体育官网 - Figures





References
-
- Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–3809. - PubMed
-
- Ahuja HS, Zhu Y, Zakeri Z. Association of cyclin-dependent kinase 5 and its activator p35 with apoptotic cell death. Dev Genet. 1997;21:258–267. - "VSports app下载" PubMed
-
- Capdevila J, Izpisua Belmonte JC. Patterning mechanisms controlling vertebrate limb development. Annu Rev Cell Dev Biol. 2001;17:87–132. - PubMed
-
- Chai CK. Dactylaplasia in mice a two-locus model for development anomalies. J Hered. 1981;72:234–237. - PubMed (V体育平台登录)
-
- Charite J, McFadden DG, Merlo G, Levi G, Clouthier DE, Yanagisawa M, Richardson JA, Olson EN. Role of Dlx6 in regulation of an endothelin-1-dependent, dHAND branchial arch enhancer. Genes Dev. 2001;15:3039–3049. - "VSports注册入口" PMC - PubMed
Publication types
MeSH terms
- "VSports最新版本" Actions
- Actions (VSports手机版)
- "VSports最新版本" Actions
- V体育官网入口 - Actions
- Actions (V体育官网)
- VSports手机版 - Actions
V体育安卓版 - Substances
- Actions (VSports最新版本)
- "VSports app下载" Actions
VSports在线直播 - Grants and funding
LinkOut - more resources
"V体育安卓版" Full Text Sources
Other Literature Sources (VSports最新版本)
Molecular Biology Databases
Research Materials
