c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8(+) T cell activation
- PMID: 11927626
- PMCID: PMC2193724
- DOI: 10.1084/jem.20011508
VSports在线直播 - c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8(+) T cell activation
Abstract
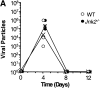
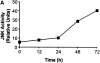
The c-Jun NH(2)-terminal kinase (JNK) signaling pathway is induced by cytokines and stress stimuli and is implicated in cell death and differentiation, but the specific function of this pathway depends on the cell type. Here we examined the role of JNK1 and JNK2 in CD8(+) T cells. Unlike CD4(+) T cells, the absence of JNK2 causes increased interleukin (IL)-2 production and proliferation of CD8(+) T cells. In contrast, JNK1-deficient CD8(+) T cells are unable to undergo antigen-stimulated expansion in vitro, even in the presence of exogenous IL-2. The hypoproliferation of these cells is associated with impaired IL-2 receptor alpha chain (CD25) gene and cell surface expression VSports手机版. The reduced level of nuclear activating protein 1 (AP-1) complexes in activated JNK1-deficient CD8(+) T cells can account for the impaired IL-2 receptor alpha chain gene expression. Thus, JNK1 and JNK2 play different roles during CD8(+) T cell activation and these roles differ from those in CD4(+) T cells. .
Figures (V体育ios版)





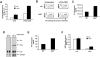
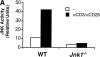
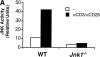
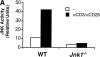
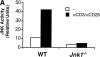


References
-
- Stenger, S., J.-P. Rosat, B. Bloom, A. Krensky, and R. Modlin. 1999. Granulysin: a lethal weapon of cytolytic T cells. Immunol. Today. 20:390–394. - PubMed
-
- Ip, Y.T., and R.J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK) - from inflammation to development. Curr. Opin. Cell Biol. 10:205–219. - VSports手机版 - PubMed
-
- Whitmarsh, A.J., and R.J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinases signal transduction pathways. J. Mol. Med. 74:589–607. - PubMed (V体育2025版)
-
- Davis, R.J. 2000. Signal transduction by the JNK group of MAP kinases. Cell. 103:239–252. - V体育官网入口 - PubMed
-
- Boulton, T.G., G.D. Yancopoulos, J.S. Gregory, C. Slaughter, C. Moomaw, J. Hsu, and M.H. Cobb. 1990. An insulin-stimulated protein kinase similar to yeast kinase involved in cell cycle control. Science. 249:64–67. - PubMed
Publication types
"V体育ios版" MeSH terms
- V体育安卓版 - Actions
- Actions (V体育平台登录)
- Actions (VSports)
- "VSports手机版" Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- "V体育安卓版" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
- Actions (VSports app下载)
Substances
- Actions (V体育平台登录)
- Actions (V体育ios版)
- "VSports最新版本" Actions
LinkOut - more resources (VSports在线直播)
Full Text Sources
Other Literature Sources
Molecular Biology Databases
"V体育官网入口" Research Materials
Miscellaneous

