"V体育平台登录" c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 signaling pathways have divergent roles in CD8(+) T cell-mediated antiviral immunity
- PMID: 11927625
- PMCID: PMC2193720
- DOI: V体育ios版 - 10.1084/jem.20011481
c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 signaling pathways have divergent roles in CD8(+) T cell-mediated antiviral immunity
Abstract
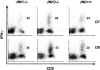
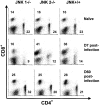
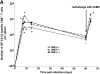
c-Jun NH(2)-terminal kinases (JNK) play important roles in T helper cell (Th) proliferation, differentiation, and maintenance of Th1/Th2 polarization. To determine whether JNKs are involved in antiviral T cell immunity, and whether JNK1 and JNK2 bear biological differences, we investigated the immune responses of JNK1-deficient and JNK2-deficient mice to lymphocytic choriomeningitis virus (LCMV). After LCMV infection, wild-type (JNK(+/+)) mice had a 5- to 10-fold increase in splenic CD8(+) T cells. In contrast, infected JNK1(-/-) mice showed a significantly lower virus-specific CD8(+) T cell expansion. However, JNK1(-/-) mice cleared LCMV infection with similar kinetics as JNK(+/+) mice VSports手机版. Splenic T cells from LCMV-infected JNK1(-/-) animals produced interferon gamma after stimulation with viral peptides. However, fewer JNK1(-/-) T cells acquired an activated phenotype (CD44(hi)) and more JNK1(-/-)CD8(+)CD44(hi) cells underwent apoptosis than JNK(+/+) cells at the peak of the primary response. In contrast, LCMV-infected JNK2(-/-) mice generated more virus-specific CD8(+) T cells than JNK(+/+) mice. These results indicate that JNK1 and JNK2 signal pathways have distinct roles in T cell responses during a viral infection. JNK1 is involved in survival of activated T cells during immune responses, and JNK2 plays a role in control of CD8(+) T cell expansion in vivo. .
Figures


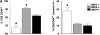
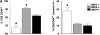

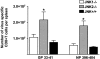
V体育安卓版 - References
-
- Whitmarsh, A.J., and R.J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589–607. - "V体育官网入口" PubMed
-
- Ip, Y.T., and R.J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol. 10:205–219. - PubMed (V体育平台登录)
-
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature. 410:37–40. - PubMed
-
- Chow, C.W., M. Rincon, J. Cavanagh, M. Dickens, and R.J. Davis. 1997. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 278:1638–1641. - PubMed (V体育ios版)
Publication types
MeSH terms
- Actions (V体育安卓版)
- Actions (VSports最新版本)
- V体育官网 - Actions
- V体育官网入口 - Actions
- Actions (VSports)
- "VSports最新版本" Actions
- Actions (VSports app下载)
"V体育官网入口" Substances
"VSports app下载" Grants and funding
"V体育ios版" LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

