Arginine-143 of Yersinia enterocolitica YopP crucially determines isotype-related NF-kappaB suppression and apoptosis induction in macrophages
- PMID: 11705945
- PMCID: PMC98859
- DOI: "VSports注册入口" 10.1128/IAI.69.12.7652-7662.2001
VSports注册入口 - Arginine-143 of Yersinia enterocolitica YopP crucially determines isotype-related NF-kappaB suppression and apoptosis induction in macrophages
Abstract
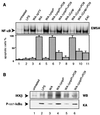
Pathogenic Yersinia spp. counteract host defense mechanisms by modulating the cellular signal relay in response to infection. Subversion of the antiapoptotic NF-kappaB signaling pathway by the Yersinia enterocolitica virulence protein YopP crucially determines the induction of apoptosis in Yersinia-infected macrophages. Here, we analyzed a panel of pathogenic, phylogenetically distinct Y. enterocolitica serotypes for their abilities to trigger macrophage apoptosis. Y. enterocolitica from the highly pathogenic serogroup O8 was substantially more effective in apoptosis induction than Yersinia from the serogroups O3 and O9. Complementation of yopP-knockout mutants revealed that this effect was specifically conferred by the serogroup O8 YopP. The amino acid sequences of YopPO8 and YopPO9 share 94% identity, and both YopP isotypes were found to interact with the NF-kappaB-activating kinase IKKbeta in macrophages. However, selectively, YopPO8 mediated efficient inhibition of IKKbeta activities, which led to substantial suppression of NF-kappaB activation. To localize the YopPO8-related effector domain, we interchanged stretches of amino acids and single amino acid residues between YopPO8 and YopPO9 VSports手机版. Functional characterization of the resulting mutants revealed a major role of the arginine-143 residue in determining the inhibitory impact of YopP on IKKbeta activity and survival of macrophages. .
Figures



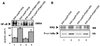

References
-
- Baichwal V R, Baeuerle P A. Apoptosis: activate NF-κB or die? Curr Biol. 1997;7:R94–R96. - PubMed (V体育ios版)
-
- Bliska J B. Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol. 2000;8:205–208. - PubMed
Publication types
V体育ios版 - MeSH terms
- "VSports" Actions
- VSports注册入口 - Actions
- V体育ios版 - Actions
- "V体育官网" Actions
- Actions (V体育ios版)
- V体育官网入口 - Actions
- VSports app下载 - Actions
- "V体育ios版" Actions
- VSports最新版本 - Actions
- VSports注册入口 - Actions
Substances
- Actions (V体育ios版)
- Actions (VSports在线直播)
- Actions (V体育ios版)
- VSports在线直播 - Actions
- Actions (V体育2025版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

