Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion (VSports注册入口)
- PMID: 11694599
- PMCID: PMC60286
- DOI: 10.1091/mbc.12.11.3690
Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion
Abstract
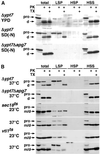
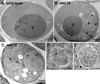
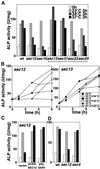
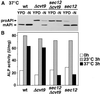
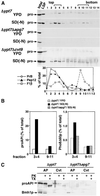
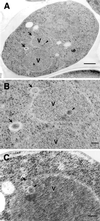
Double membrane structure, autophagosome, is formed de novo in the process of autophagy in the yeast Saccharomyces cerevisiae, and many Apg proteins participate in this process. To further understand autophagy, we analyzed the involvement of factors engaged in the secretory pathway VSports手机版. First, we showed that Sec18p (N-ethylmaleimide-sensitive fusion protein, NSF) and Vti1p (soluble N-ethylmaleimide-sensitive fusion protein attachment protein, SNARE), and soluble N-ethylmaleimide-sensitive fusion protein receptor are required for fusion of the autophagosome to the vacuole but are not involved in autophagosome formation. Second, Sec12p was shown to be essential for autophagy but not for the cytoplasm to vacuole-targeting (Cvt) (pathway, which shares mostly the same machinery with autophagy. Subcellular fractionation and electron microscopic analyses showed that Cvt vesicles, but not autophagosomes, can be formed in sec12 cells. Three other coatmer protein (COPII) mutants, sec16, sec23, and sec24, were also defective in autophagy. The blockage of autophagy in these mutants was not dependent on transport from endoplasmic reticulum-to-Golgi, because mutations in two other COPII genes, SEC13 and SEC31, did not affect autophagy. These results demonstrate the requirement for subgroup of COPII proteins in autophagy. This evidence demonstrating the involvement of Sec proteins in the mechanism of autophagosome formation is crucial for understanding membrane flow during the process. .
Figures



"VSports最新版本" References
-
- Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997.
-
- Barlowe C, Orc L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Scheckman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed (V体育官网)
Publication types
- Actions (V体育官网)
MeSH terms
- "V体育官网入口" Actions
- "VSports手机版" Actions
- V体育ios版 - Actions
- "V体育ios版" Actions
- Actions (V体育平台登录)
- V体育安卓版 - Actions
- V体育2025版 - Actions
- "VSports手机版" Actions
- "V体育ios版" Actions
- VSports - Actions
- V体育安卓版 - Actions
- Actions (V体育官网)
VSports手机版 - Substances
- Actions (V体育安卓版)
- VSports在线直播 - Actions
- Actions (VSports最新版本)
- "V体育ios版" Actions
- "VSports在线直播" Actions
- V体育2025版 - Actions
- Actions (VSports最新版本)
- "VSports" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

