The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis (V体育2025版)
- PMID: 11595797
- PMCID: PMC139154
- DOI: 10.1105/tpc.010085
The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis
Abstract (V体育官网入口)
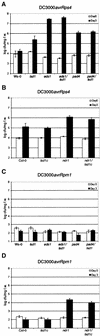
Specific recognition of pathogens is mediated by plant disease resistance (R) genes and translated into a successful defense response. The extent of associated hypersensitive cell death varies from none to an area encompassing cells surrounding an infection site, depending on the R gene activated. We constructed double mutants in Arabidopsis between positive regulators of R function and a negative regulator of cell death, LSD1, to address whether genes required for normal R function also regulate the runaway cell death observed in lsd1 mutants. We report here that EDS1 and PAD4, two signaling genes that mediate some but not all R responses, also are required for runaway cell death in the lsd1 mutant. Importantly, this novel function of EDS1 and PAD4 is operative when runaway cell death in lsd1 is initiated through an R gene that does not require EDS1 or PAD4 for disease resistance. NDR1, another component of R signaling, also contributes to the control of plant cell death. The roles of EDS1 and PAD4 in regulating lsd1 runaway cell death are related to the interpretation of reactive oxygen intermediate-derived signals at infection sites. We further demonstrate that the fate of superoxide at infection sites is different from that observed at the leading margins of runaway cell death lesions in lsd1 mutants. VSports手机版.
Figures





References
-
- Aarts, N., Metz, M., Holub, E., Staskawicz, B.J., Daniels, M.J., and Parker, J.E. (1998). Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene–mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 10306–10311. - "VSports app下载" PMC - PubMed
-
- Baker, C.J., and Orlandi, E.W. (1995). Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33, 299–321. - PubMed
-
- Bolwell, G.P. (1999). Role of active oxygen species and NO in plant defense responses. Curr. Opin. Plant Biol. 2, 287–294. - PubMed
Publication types
MeSH terms
- V体育2025版 - Actions
- "VSports注册入口" Actions
- "VSports最新版本" Actions
- V体育官网入口 - Actions
- "V体育平台登录" Actions
- VSports app下载 - Actions
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- Actions (V体育ios版)
- VSports注册入口 - Actions
Substances
- Actions (V体育平台登录)
- "VSports手机版" Actions
- V体育ios版 - Actions
Grants and funding
VSports注册入口 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

