Role of p14(ARF) in replicative and induced senescence of human fibroblasts
- PMID: 11564860
- PMCID: PMC99853
- DOI: 10.1128/MCB.21.20.6748-6757.2001
Role of p14(ARF) in replicative and induced senescence of human fibroblasts (VSports手机版)
Abstract
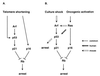
Following a proliferative phase of variable duration, most normal somatic cells enter a growth arrest state known as replicative senescence VSports手机版. In addition to telomere shortening, a variety of environmental insults and signaling imbalances can elicit phenotypes closely resembling senescence. We used p53(-/-) and p21(-/-) human fibroblast cell strains constructed by gene targeting to investigate the involvement of the Arf-Mdm2-p53-p21 pathway in natural as well as premature senescence states. We propose that in cell types that upregulate p21 during replicative exhaustion, such as normal human fibroblasts, p53, p21, and Rb act sequentially and constitute the major pathway for establishing growth arrest and that the telomere-initiated signal enters this pathway at the level of p53. Our results also revealed a number of significant differences between human and rodent fibroblasts in the regulation of senescence pathways. .
VSports手机版 - Figures



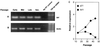

References
-
- Artandi S E, DePinho R A. Mice without telomerase: what can they teach us about human cancer? Nat Med. 2000;6:852–855. - PubMed
-
- Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. - "VSports最新版本" PubMed
VSports手机版 - Publication types
- Actions (V体育平台登录)
MeSH terms
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- VSports - Actions
- "V体育安卓版" Actions
- V体育ios版 - Actions
- Actions (V体育官网)
- V体育平台登录 - Actions
- "V体育安卓版" Actions
- Actions (VSports app下载)
- "V体育ios版" Actions
Substances (V体育ios版)
- "VSports在线直播" Actions
- VSports - Actions
- Actions (V体育安卓版)
- "V体育官网入口" Actions
Grants and funding
LinkOut - more resources (VSports注册入口)
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous (V体育2025版)
