Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection
- PMID: 11544273
- PMCID: PMC209384
- DOI: 10.1172/JCI12976
Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection
Abstract
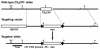
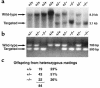
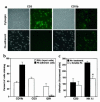
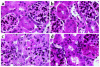
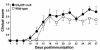
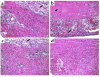
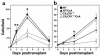
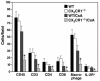
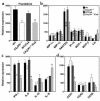
Fractalkine (Fk) is a structurally unusual member of the chemokine family. To determine its role in vivo, we generated mice with a targeted disruption of CX(3)CR1, the receptor for Fk. CX(3)CR1(-/-) mice were phenotypically indistinguishable from wild-type mice in a pathogen-free environment. In response to antibody-induced glomerulonephritis, CX(3)CR1(-/-) and CX(3)CR1(+/+) mice had similar levels of proteinuria and injury. CX(3)CR1(-/-) and CX(3)CR1(+/+) mice also developed similar levels of disease in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. We performed heterotopic MHC class I/II cardiac transplants from BALB/c mice into C57BL/6 mice VSports手机版. In the absence of cyclosporin A (CsA), there was no difference in graft survival time between CX(3)CR1(-/-) and CX(3)CR1(+/+) recipient mice. However, in the presence of subtherapeutic levels of CsA, graft survival time was significantly increased in the CX(3)CR1(-/-) mice. Characterization of cells infiltrating the grafts revealed a selective reduction in natural killer cells in the CX(3)CR1(-/-) recipients in the absence of CsA and a reduction in macrophages, natural killer cells, and other leukocytes in the presence of CsA. We conclude that Fk plays an important role in graft rejection. The development of CX(3)CR1 antagonists may allow reductions in the doses of immunosuppressive drugs used in transplantation. .
Figures
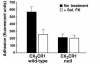


References (VSports app下载)
-
- Bazan JF, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. - PubMed
-
- Imai T, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. - "VSports" PubMed
-
- Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–10058. - PubMed
-
- Fong AM, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. - VSports手机版 - PMC - PubMed
-
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. - PubMed
Publication types
- "V体育官网" Actions
MeSH terms
- Actions (VSports)
- VSports手机版 - Actions
- Actions (V体育安卓版)
- Actions (VSports手机版)
- Actions (VSports在线直播)
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- V体育ios版 - Actions
- V体育2025版 - Actions
- Actions (V体育官网入口)
- V体育安卓版 - Actions
Substances
- "VSports手机版" Actions
- "VSports" Actions
- Actions (VSports)
- Actions (V体育官网)
- V体育安卓版 - Actions
- V体育官网入口 - Actions
- Actions (VSports在线直播)
"VSports注册入口" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
V体育官网入口 - Research Materials

