CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity
- PMID: 11401974
- PMCID: "VSports" PMC98507
- DOI: 10.1128/IAI.69.7.4358-4365.2001
CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity
"VSports" Abstract
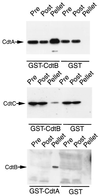
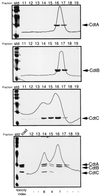
Campylobacter jejuni encodes a cytolethal distending toxin (CDT) that causes cells to arrest in the G(2)/M transition phase of the cell cycle. Highly related toxins are also produced by other important bacterial pathogens VSports手机版. CDT activity requires the function of three genes: cdtA, cdtB, and cdtC. Recent studies have established that CdtB is the active subunit of CDT, exerting its effect as a nuclease that damages the DNA and triggers cell cycle arrest. Microinjection of CdtB into target cells led to G(2)/M arrest and cytoplasmic distention, in a manner indistinguishable from that caused by CDT treatment. Despite this progress, nothing is known about the composition of the CDT holotoxin or the function of CdtA and CdtC. We show here that, when applied individually, purified CdtA, CdtB, or CdtC does not exhibit toxic activity. In contrast, CdtA, CdtB, and CdtC when combined, interact with one another to form an active tripartite holotoxin that exhibits full cellular toxicity. CdtA has a domain that shares similarity with the B chain of ricin-related toxins. We therefore proposed that CDT is a tripartite toxin composed of CdtB as the enzymatically active subunit and of CdtA and CdtC as the heterodimeric B subunit required for the delivery of CdtB. .
Figures

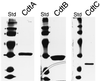


References
-
- Collazo C M, Galan J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. - PMC (VSports手机版) - PubMed
-
- Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. - VSports在线直播 - PMC - PubMed
MeSH terms
- Actions (VSports)
- "V体育安卓版" Actions
- VSports - Actions
- Actions (VSports在线直播)
- Actions (V体育ios版)
Substances
- Actions (V体育官网)
- Actions (VSports注册入口)
LinkOut - more resources
Full Text Sources

