V体育平台登录 - Genetic evidence for the involvement of DNA ligase IV in the DNA-PK-dependent pathway of non-homologous end joining in mammalian cells
- PMID: 11292837
- PMCID: PMC31316
- DOI: 10.1093/nar/29.8.1653
Genetic evidence for the involvement of DNA ligase IV in the DNA-PK-dependent pathway of non-homologous end joining in mammalian cells
Abstract
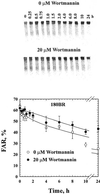
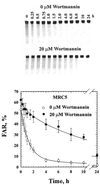
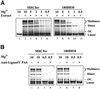
Cells of vertebrates remove DNA double-strand breaks (DSBs) from their genome predominantly utilizing a fast, DNA-PKcs-dependent form of non-homologous end joining (D-NHEJ). Mutants with inactive DNA-PKcs remove the majority of DNA DSBs utilizing a slow, DNA-PKcs-independent pathway that does not utilize genes of the RAD52 epistasis group, is error-prone and can therefore be classified as a form of NHEJ (termed basic or B-NHEJ). We studied the role of DNA ligase IV in these pathways of NHEJ. Although biochemical studies show physical and functional interactions between the DNA-PKcs/Ku and the DNA ligase IV/Xrcc4 complexes suggesting operation within the same pathway, genetic evidence to support this notion is lacking in mammalian cells. Primary human fibroblasts (180BR) with an inactivating mutation in DNA ligase IV, rejoined DNA DSBs predominantly with slow kinetics similar to those observed in cells deficient in DNA-PKcs, or in wild-type cells treated with wortmannin to inactivate DNA-PK. Treatment of 180BR cells with wortmannin had only a small effect on DNA DSB rejoining and no effect on cell radiosensitivity to killing although it sensitized control cells to 180BR levels. This is consistent with DNA ligase IV functioning as a component of the D-NHEJ, and demonstrates the unperturbed operation of the DNA-PKcs-independent pathway (B-NHEJ) at significantly reduced levels of DNA ligase IV. In vitro, extracts of 180BR cells supported end joining of restriction endonuclease-digested plasmid to the same degree as extracts of control cells when tested at 10 mM Mg(2+). At 0 VSports手机版. 5 mM Mg(2+), where only DNA ligase IV is expected to retain activity, low levels of end joining ( approximately 10% of 10 mM) were seen in the control but there was no detectable activity in 180BR cells. Antibodies raised against DNA ligase IV did not measurably inhibit end joining at 10 mM Mg(2+) in either cell line. Thus, in contrast to the situation in vivo, end joining in vitro is dominated by pathways with properties similar to B-NHEJ that do not display a strong dependence on DNA ligase IV, with D-NHEJ retaining only a limited contribution. The implications of these observations to studies of NHEJ in vivo and in vitro are discussed. .
Figures

"VSports" References
-
- DiBiase S.J., Zeng,Z.-C., Chen,R., Hyslop,T., Curran,W.J.,Jr and Iliakis,G. (2000) DNA-dependent protein kinase stimulates an independently active, nonhomologous, end-joining apparatus. Cancer Res., 60, 1245–1253. - PubMed
-
- Thacker J. (1999) A surfeit of RAD51-like genes? Trends Genet., 15, 166–168. - PubMed
-
- Thompson, L.H. and Schild,D. (1999) The contribution of homologous recombination in preserving genome integrity in mammalian cells. Biochimie, 81, 87–105. - PubMed (V体育ios版)
-
- Jeggo P.A. (1998) DNA breakage and repair. Adv. Genet., 38, 186–218. - PubMed
-
- Wang H., Zeng,Z.-C., Bui,T.-A., Sonoda,E., Takata,M., Takeda,S. and Iliakis,G. (2001) Efficient rejoining of radiation-induced DNA double-strand breaks in vertebrate cells deficient in genes of the RAD52 epistasis group. Oncogene, in press. - PubMed
"V体育平台登录" Publication types
MeSH terms
- "V体育官网入口" Actions
- "VSports" Actions
- Actions (V体育安卓版)
- "V体育ios版" Actions
- VSports在线直播 - Actions
- V体育官网 - Actions
- VSports - Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
- VSports app下载 - Actions
- V体育ios版 - Actions
- VSports在线直播 - Actions
- "VSports最新版本" Actions
- VSports注册入口 - Actions
- "VSports手机版" Actions
- "VSports最新版本" Actions
- VSports在线直播 - Actions
"VSports app下载" Substances
- VSports - Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- "VSports最新版本" Actions
- V体育2025版 - Actions
- "V体育官网入口" Actions
- Actions (V体育2025版)
Grants and funding
LinkOut - more resources (VSports在线直播)
Full Text Sources (VSports最新版本)
Research Materials (VSports注册入口)

