VSports最新版本 - Molecular basis for immunoglobulin M specificity to epitopes in Cryptococcus neoformans polysaccharide that elicit protective and nonprotective antibodies
- PMID: 11292763
- PMCID: PMC98299
- DOI: 10.1128/IAI.69.5.3398-3409.2001
Molecular basis for immunoglobulin M specificity to epitopes in Cryptococcus neoformans polysaccharide that elicit protective and nonprotective antibodies
Abstract
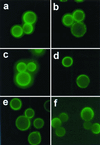
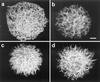
The protective efficacy of antibodies (Abs) to Cryptococcus neoformans glucuronoxylomannan (GXM) is dependent on Ab fine specificity. Two clonally related immunoglobulin M monoclonal Abs (MAbs) (12A1 and 13F1) differ in fine specificity and protective efficacy, presumably due to variable (V)-region sequence differences resulting from somatic mutations. MAb 12A1 is protective and produces annular immunofluorescence (IF) on serotype D C. neoformans, while MAb 13F1 is not protective and produces punctate IF. To determine the Ab molecular determinants responsible for the IF pattern, site-directed mutagenesis of the MAb 12A1 heavy-chain V region (V(H)) was followed by serological and functional studies of the various mutants. Changing two selected amino acids in the 12A1 V(H) binding cavity to the corresponding residues in the 13F1 V(H) altered the IF pattern from annular to punctate, reduced opsonic efficacy, and abolished recognition by an anti-idiotypic Ab. Analysis of the binding of the various mutants to peptide mimetics revealed that different amino acids were responsible for GXM binding and peptide specificity. The results suggest that V-region motifs associated with annular binding and opsonic activity may be predictive of Ab efficacy against C. neoformans. This has important implications for immunotherapy and vaccine design that are reinforced by the finding that GXM and peptide reactivities are determined by different amino acid residues. VSports手机版.
Figures



References
-
- Beenhouwer D O, Valadon P, May R, Scharff M D. Peptide mimicry of the polysaccharide capsule of Cryptococcus neoformans. In: Cunningham M W, Fujinami R S, editors. Molecular mimicry, microbes, and autoimmunity. Washington, D.C.: ASM Press; 2000. pp. 143–160.
-
- Brorson K, Thompson C, Wei G, Krasnokutsky M, Stein K E. Mutational analysis of avidity and fine specificity of anti-levan antibodies. J Immunol. 1999;163:6694–6701. - PubMed
-
- Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukherjee J, Pirofski L, Rivera J, Rosas A L, Scharff M D, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. - PMC - PubMed
Publication types
- "VSports最新版本" Actions
- Actions (V体育2025版)
MeSH terms
- Actions (V体育ios版)
- "V体育官网" Actions
- VSports app下载 - Actions
- Actions (V体育安卓版)
- "VSports最新版本" Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "VSports最新版本" Actions
- Actions (V体育平台登录)
- Actions (VSports)
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

