The molecular basis of human 3-methylcrotonyl-CoA carboxylase deficiency
- PMID: 11181649
- PMCID: PMC199271
- DOI: "VSports最新版本" 10.1172/JCI11948
The molecular basis of human 3-methylcrotonyl-CoA carboxylase deficiency
Abstract (V体育安卓版)
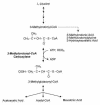
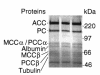
Isolated biotin-resistant 3-methylcrotonyl-CoA carboxylase (MCC) deficiency is an autosomal recessive disorder of leucine catabolism that appears to be the most frequent organic aciduria detected in tandem mass spectrometry-based neonatal screening programs. The phenotype is variable, ranging from neonatal onset with severe neurological involvement to asymptomatic adults VSports手机版. MCC is a heteromeric mitochondrial enzyme composed of biotin-containing alpha subunits and smaller beta subunits. Here, we report cloning of MCCA and MCCB cDNAs and the organization of their structural genes. We show that a series of 14 MCC-deficient probands defines two complementation groups, CG1 and 2, resulting from mutations in MCCB and MCCA, respectively. We identify five MCCA and nine MCCB mutant alleles and show that missense mutations in each result in loss of function. .
Figures



References
-
- Sweetman, L., and Williams, J.C. 1995. Branched chain organic acidurias. In The metabolic and molecular bases of inherited disease. 7th edition. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 1387–1422.
-
- Bannwart C, Wermuth B, Baumgartner R, Suormala T, Weismann UN. Isolated biotin-resistant deficiency of 3-methylcrotonyl-CoA carboxylase presenting as a clinically severe form in a newborn with fatal outcome. J Inherit Metab Dis. 1992; 15:863–868. - PubMed
-
- Lehnert W, Niederhoff H, Suormala T, Baumgartner ER. Isolated biotin-resistant 3-methylcrotonyl-CoA carboxylase deficiency: long-term outcome in a case with neonatal onset. Eur J Pediatr. 1996; 155:568–572. - PubMed
-
- Gibson KM, Bennett MJ, Naylor EW, Morton DH. 3-Methylcrotonyl-coenzyme A carboxylase deficiency in Amish/Mennonite adults identified by detection of increased acylcarnitines in blood spots of their children. J Pediatr. 1998; 132:519–523. - PubMed
-
- Levy HL. Newborn screening by tandem mass spectrometry: a new era. Clin Chem. 1998; 44:2401–2402. - PubMed
Publication types
MeSH terms (V体育ios版)
- "VSports手机版" Actions
- "V体育ios版" Actions
- Actions (V体育平台登录)
- "VSports注册入口" Actions
- Actions (VSports app下载)
- VSports在线直播 - Actions
- V体育2025版 - Actions
Substances
"VSports" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (VSports)
Miscellaneous (VSports注册入口)

