DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells
- PMID: 11015446
- PMCID: PMC2193316
- DOI: 10.1084/jem.192.7.1059
DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells
Abstract
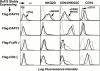
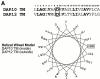
Many of the activating receptors on natural killer (NK) cells are multisubunit complexes composed of ligand-binding receptors that are noncovalently associated with membrane-bound signaling adaptor proteins, including CD3zeta, FcstraightepsilonRIgamma, DAP12, and DAP10 VSports手机版. Because the DAP10 and DAP12 genes are closely linked, expressed in NK cells, and have remarkably similar transmembrane segments, it was of interest to determine the specificity of their interactions with ligand-binding receptors and to examine their signaling properties. Despite their similarities, DAP10, DAP12, FcstraightepsilonRIgamma, and CD3zeta form specific receptor complexes with their ligand-binding partners in NK cells and transfectants. The transmembrane regions of DAP10 and DAP12 are sufficient to confer specific association with their partners. Although cross-linking of either DAP10- or DAP12-associated receptors has been shown to be sufficient to trigger NK cell-mediated cytotoxicity against Fc receptor-bearing cells, substantial synergy was observed in the induction of cytokine production when both receptors were engaged. Activation of the Syk/ZAP70 tyrosine kinases by the immunoreceptor tyrosine-based activation motif-containing DAP12 adaptor and of the phosphatidylinositol 3-kinase pathway by the YxNM-containing DAP10 adaptor may play an important role in the stimulation of NK cells and T cells. .
Figures






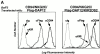
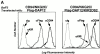






References
-
- Lanier L.L. Turning on natural killer cells. J. Exp. Med. 2000;191:1259–1262. - PMC (VSports app下载) - PubMed
-
- Long E.O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 1999;17:875–904. - PubMed
-
- Mason L.H., Willette-Brown J., Anderson S.K., Gosselin P., Shores E.W., Love P.E., Ortaldo J.R., McVicar D.W. Characterization of an associated 16 kDa tyrosine phosphoprotein required for Ly-49D signal transduction. J. Immunol. 1998;160:4148–4152. - V体育官网入口 - PubMed
-
- Olcese L., Cambiaggi A., Semenzato G., Bottino C., Moretta A., Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 1997;158:5083–5086. - PubMed
-
- Lanier L.L., Corliss B.C., Wu J., Leong C., Phillips J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. - "V体育2025版" PubMed
Publication types
- "VSports注册入口" Actions
"V体育官网" MeSH terms
- VSports手机版 - Actions
- VSports - Actions
- Actions (VSports最新版本)
- "VSports在线直播" Actions
- "V体育ios版" Actions
- V体育ios版 - Actions
- Actions (V体育2025版)
- Actions (V体育ios版)
- "VSports在线直播" Actions
- VSports app下载 - Actions
Substances
- "V体育安卓版" Actions
- V体育官网入口 - Actions
- "V体育官网" Actions
- Actions (VSports注册入口)
- V体育2025版 - Actions
- Actions (VSports)
"V体育官网" Grants and funding
LinkOut - more resources
"V体育官网" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

