Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation
- PMID: 10958690
- PMCID: PMC88770
- DOI: 10.1128/MCB.20.18.6945-6957.2000
Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation
Abstract (VSports app下载)
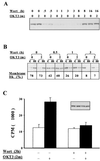
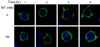
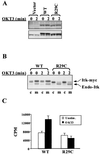
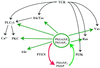
Pleckstrin homology (PH) domain binding to D3-phosphorylated phosphatidylinositides (PI) provides a reversible means of recruiting proteins to the plasma membrane, with the resultant change in subcellular localization playing a key role in the activation of multiple intracellular signaling pathways. Previously we found that the T-cell-specific PH domain-containing kinase Itk is constitutively membrane associated in Jurkat T cells. This distribution was unexpected given that the closely related B-cell kinase, Btk, is almost exclusively cytosolic. In addition to constitutive membrane association of Itk, unstimulated JTAg T cells also exhibited constitutive phosphorylation of Akt on Ser-473, an indication of elevated basal levels of the phosphatidylinositol 3-kinase (PI3K) products PI-3,4-P(2) and PI-3,4,5-P(3) in the plasma membrane. Here we describe a defect in expression of the D3 phosphoinositide phosphatase, PTEN, in Jurkat and JTAg T cells that leads to unregulated PH domain interactions with the plasma membrane VSports手机版. Inhibition of D3 phosphorylation by PI3K inhibitors, or by expression of PTEN, blocked constitutive phosphorylation of Akt on Ser-473 and caused Itk to redistribute to the cytosol. The PTEN-deficient cells were also hyperresponsive to T-cell receptor (TCR) stimulation, as measured by Itk kinase activity, tyrosine phosphorylation of phospholipase C-gamma1, and activation of Erk compared to those in PTEN-replete cells. These data support the idea that PH domain-mediated association with the plasma membrane is required for Itk activation, provide evidence for a negative regulatory role of PTEN in TCR stimulation, and suggest that signaling models based on results from Jurkat T-cell lines may underestimate the role of PI3K in TCR signaling. .
VSports注册入口 - Figures


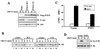


References
-
- Andjelkovic M, Maira S M, Cron P, Parker P J, Hemmings B A. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol Cell Biol. 1999;19:5061–5072. - VSports手机版 - PMC - PubMed
-
- Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. - PubMed
-
- Besson A, Robbins S M, Yong V W. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605–611. - PubMed
MeSH terms
- Actions (V体育2025版)
- Actions (VSports注册入口)
- VSports app下载 - Actions
- "VSports注册入口" Actions
- Actions (V体育官网入口)
- Actions (VSports注册入口)
- VSports app下载 - Actions
- "V体育平台登录" Actions
- Actions (VSports)
- Actions (VSports手机版)
- Actions (VSports最新版本)
- V体育官网入口 - Actions
- "V体育安卓版" Actions
Substances
- "VSports最新版本" Actions
- Actions (V体育ios版)
- Actions (VSports)
- "VSports最新版本" Actions
- VSports - Actions
- "VSports注册入口" Actions
- "VSports手机版" Actions
- Actions (VSports在线直播)
- V体育官网入口 - Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials (VSports app下载)
Miscellaneous
