Human immunodeficiency virus type 1 Vif protein is an integral component of an mRNP complex of viral RNA and could be involved in the viral RNA folding and packaging process
- PMID: 10954522
- PMCID: "V体育2025版" PMC116333
- DOI: V体育安卓版 - 10.1128/jvi.74.18.8252-8261.2000
Human immunodeficiency virus type 1 Vif protein is an integral component of an mRNP complex of viral RNA and could be involved in the viral RNA folding and packaging process
Abstract
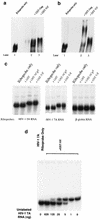
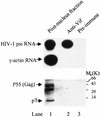
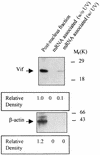
Virion infectivity factor (Vif) is a protein encoded by human immunodeficiency virus types 1 and 2 (HIV-1 and -2) and simian immunodeficiency virus, plus other lentiviruses, and is essential for viral replication either in vivo or in culture for nonpermissive cells such as peripheral blood lymphoid cells, macrophages, and H9 T cells. Defects in the vif gene affect virion morphology and reverse transcription but not the expression of viral components VSports手机版. It has been shown that Vif colocalizes with Gag in cells and Vif binds to the NCp7 domain of Gag in vitro. However, it seems that Vif is not specifically packaged into virions. The molecular mechanism(s) for Vif remains unknown. In this report, we demonstrate that HIV-1 Vif is an RNA-binding protein and specifically binds to HIV-1 genomic RNA in vitro. Further, Vif binds to HIV-1 RNA in the cytoplasm of virus-producing cells to form a 40S mRNP complex. Coimmunoprecipitation and in vivo UV cross-linking assays indicated that Vif directly interact with HIV-1 RNA in the virus-producing cells. Vif-RNA binding could be displaced by Gag-RNA binding, suggesting that Vif protein in the mRNP complex may mediate viral RNA interaction with HIV-1 Gag precursors. Furthermore, we have demonstrated that these Vif mutants that lose the RNA binding activity in vitro do not support vif-deficient HIV-1 replication in H9 T cells, suggesting that the RNA binding capacity of Vif is important for its function. Further studies regarding Vif-RNA interaction in virus-producing cells will be important for studying the function of Vif in the HIV-1 life cycle. .
Figures
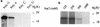




References
-
- Afonina E, Stauber R, Pavlakis G N. The human poly(A)-binding protein 1 shuttles between the nucleus and the cytoplasm. J Biol Chem. 1998;273:13015–13021. - "VSports注册入口" PubMed
-
- Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. - PubMed
"V体育官网入口" Publication types
- "VSports注册入口" Actions
MeSH terms
- Actions (V体育平台登录)
- VSports - Actions
- Actions (VSports手机版)
- Actions (V体育ios版)
- VSports在线直播 - Actions
- "VSports" Actions
- "V体育官网入口" Actions
VSports手机版 - Substances
- V体育安卓版 - Actions
- Actions (VSports在线直播)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

