Internalization of CD26 by mannose 6-phosphate/insulin-like growth factor II receptor contributes to T cell activation
- PMID: 10900005
- PMCID: PMC26966
- DOI: 10.1073/pnas.97.15.8439 (V体育官网入口)
Internalization of CD26 by mannose 6-phosphate/insulin-like growth factor II receptor contributes to T cell activation
"V体育安卓版" Abstract
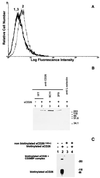
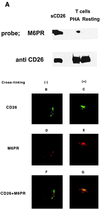
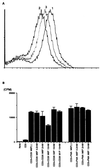
CD26 is a T cell activation antigen known to bind adenosine deaminase and have dipeptidyl peptidase IV activity. Cross-linking of CD26 and CD3 with immobilized mAbs can deliver a costimulatory signal that contributes to T cell activation VSports手机版. Our earlier studies revealed that cross-linking of CD26 induces its internalization, the phosphorylation of a number of proteins involved in the signaling pathway, and subsequent T cell proliferation. Although these findings suggest the importance of internalization in the function of CD26, CD26 has only 6 aa residues in its cytoplasmic region with no known motif for endocytosis. In the present study, we have identified the mannose 6-phosphate/insulin-like growth factor II receptor (M6P/IGFIIR) as a binding protein for CD26 and that mannose 6-phosphate (M6P) residues in the carbohydrate moiety of CD26 are critical for this binding. Activation of peripheral blood T cells results in the mannose 6 phosphorylation of CD26. In addition, the cross-linking of CD26 with an anti-CD26 antibody induces not only capping and internalization of CD26 but also colocalization of CD26 with M6P/IGFIIR. Finally, both internalization of CD26 and the T cell proliferative response induced by CD26-mediated costimulation were inhibited by the addition of M6P, but not by glucose 6-phosphate or mannose 1-phosphate. These results indicate that internalization of CD26 after cross-linking is mediated in part by M6P/IGFIIR and that the interaction between mannose 6-phosphorylated CD26 and M6P/IGFIIR may play an important role in CD26-mediated T cell costimulatory signaling. .
Figures
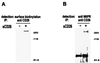

"V体育官网" References
-
- Morimoto C, Torimoto Y, Levinson G, Rudd C E, Schrieber M, Dang N H, Letvin N L, Schlossman S F. J Immunol. 1989;143:3430–3439. - PubMed
-
- Morimoto C, Schlossman S F. Immunol Rev. 1998;161:55–70. - PubMed
-
- Hegen M, Niedobitek G, Klein C E, Stein H, Fleischer B. J Immunol. 1990;144:2908–2914. - PubMed
-
- Torimoto Y, Dang N H, Tanaka T, Prado C, Schlossman S F, Morimoto C. Mol Immunol. 1992;29:183–192. - PubMed
Publication types
- "V体育2025版" Actions
MeSH terms
- Actions (VSports注册入口)
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- "VSports" Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- "VSports app下载" Actions
"VSports app下载" Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
"VSports在线直播" Miscellaneous

