"V体育官网入口" c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1
- PMID: 10866678
- PMCID: PMC85971
- DOI: VSports注册入口 - 10.1128/MCB.20.14.5227-5234.2000
V体育平台登录 - c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1
Abstract
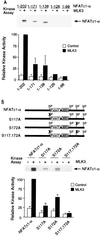
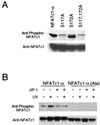
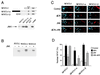
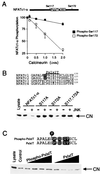
The protein phosphatase calcineurin is a critical mediator of calcium signals during T-cell activation. One substrate of calcineurin is the transcription factor NFATc1, which is retained in the cytoplasm of quiescent cells. NFATc1 activation requires the translocation of the transcription factor into the nucleus, a process that is mediated by calcineurin. This interaction with calcineurin requires a targeting domain (PxIxIT motif) located in the NH(2)-terminal region of NFATc1 VSports手机版. Here we demonstrate that the calcineurin targeting domain of NFATc1 is phosphorylated and inactivated by the c-Jun NH(2)-terminal kinase (JNK). This disruption of calcineurin targeting inhibits the nuclear accumulation and transcription activity of NFATc1 and accounts for the observation that Jnk1(-/-) T cells exhibit greatly increased NFATc1-dependent nuclear responses. .
"VSports app下载" Figures
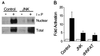


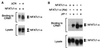
References
-
- Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan P G. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. - PubMed (V体育2025版)
-
- Aramburu J, Yaffe M B, Lopez-Rodriguez C, Cantley L C, Hogan P G, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. - PubMed
-
- Avots A, Buttmann M, Chuvpilo S, Escher C, Smola U, Bannister A J, Rapp U R, Kouzarides T, Serfling E. CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity. 1999;10:515–524. - "VSports最新版本" PubMed
-
- Beals C R, Clipstone N A, Ho S N, Crabtree G R. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997;11:824–834. - PubMed
-
- Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. - "VSports在线直播" PubMed
Publication types
"VSports最新版本" MeSH terms
- "V体育安卓版" Actions
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- Actions (VSports)
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- VSports在线直播 - Actions
- Actions (V体育安卓版)
- V体育2025版 - Actions
- V体育官网入口 - Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
- Actions (VSports在线直播)
- Actions (VSports注册入口)
Substances
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- Actions (VSports app下载)
- "VSports" Actions
- VSports注册入口 - Actions
- "VSports注册入口" Actions
- "VSports" Actions
Grants and funding
"V体育平台登录" LinkOut - more resources
Full Text Sources (VSports最新版本)
Other Literature Sources
Molecular Biology Databases
Research Materials
"V体育ios版" Miscellaneous
