Glutathione synthesis is essential for mouse development but not for cell growth in culture
- PMID: 10805773
- PMCID: VSports在线直播 - PMC25788
- DOI: 10.1073/pnas.97.10.5101
Glutathione synthesis is essential for mouse development but not for cell growth in culture
Abstract
Glutathione (GSH) is a major source of reducing equivalents in mammalian cells. To examine the role of GSH synthesis in development and cell growth, we generated mice deficient in GSH by a targeted disruption of the heavy subunit of gamma-glutamylcysteine synthetase (gammaGCS-HS(tm1)), an essential enzyme in GSH synthesis VSports手机版. Embryos homozygous for gammaGCS-HS(tm1) fail to gastrulate, do not form mesoderm, develop distal apoptosis, and die before day 8. 5. Lethality results from apoptotic cell death rather than reduced cell proliferation. We also isolated cell lines from homozygous mutant blastocysts in medium containing GSH. These cells also grow indefinitely in GSH-free medium supplemented with N-acetylcysteine and have undetectable levels of GSH; further, they show no changes in mitochondrial morphology as judged by electron microscopy. These data demonstrate that GSH is required for mammalian development but dispensable in cell culture and that the functions of GSH, not GSH itself, are essential for cell growth. .
Figures
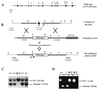

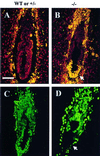
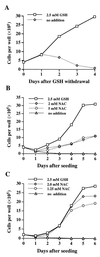
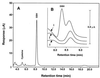
References
-
- Kosower N S. Int Rev Cytol. 1978;54:109–160. - PubMed (VSports最新版本)
-
- Meister A. J Biol Chem. 1988;263:17205–17208. - PubMed
-
- Holmgren A. In: Glutathione: Metabolism and Physiological Function. Viña J, editor. Boca Raton, FL: CRC; 1990. pp. 145–154.
-
- Prinz W A, Aslund F, Holmgren A, Beckwith J. J Biol Chem. 1997;272:15661–15667. - PubMed
Publication types
MeSH terms
- V体育ios版 - Actions
- VSports app下载 - Actions
- "V体育ios版" Actions
- VSports app下载 - Actions
- "V体育平台登录" Actions
- "VSports" Actions
- V体育ios版 - Actions
- Actions (VSports)
- Actions (V体育ios版)
- "VSports" Actions
Substances
- "V体育平台登录" Actions
- V体育官网入口 - Actions
Grants and funding
LinkOut - more resources
VSports - Full Text Sources
V体育安卓版 - Other Literature Sources
Molecular Biology Databases

